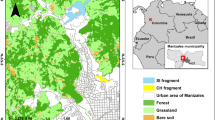
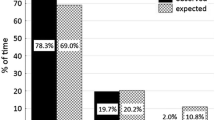
Abstract
The presence of other animals, both conspecifics and heterospecifics, is a major driving force for how animals organize themselves in space and time. Although theoretical models are available to explain the role of each in animal movement, fine-scale assessments of daily movement are scarce, particularly for primates. Hence, our goal was to assess whether and how the presence of conspecifics and heterospecifics influence spatiotemporal landscape use in two, wild, howler monkey (Alouatta guariba) groups. We followed the groups for 14 months in a large, continuous forest, during which we recorded their daily path length (DPL), home range, activity budget, feeding, and the presence of other groups (conspecifics) and other species (heterospecifics). The two groups differed in DPL, home range, proportion of fruits ingested, and time devoted to moving and resting. Partial least squares path modelling showed that variation in DPL was explained by the percentage of leaves or fruits ingested and by the presence of conspecifics, but not of heterospecifics. Group differences in several ecological variables emphasise the need to conduct further studies of space use with more groups in the same area to understand the underlying mechanisms of these differences. Moreover, our analysis shows that within-species interactions may be a stronger force in spatiotemporal organisation than interspecies interactions, at least in this folivorous primate. This is relevant from both a theoretical standpoint, and also when considering the consequences of habitat fragmentation and reduction. Deforestation leads to decreased resource availability and increased likelihood of encounters with conspecifics, which ultimately alters the proportion of food items ingested and increases the DPL, disrupting energy balance.




Similar content being viewed by others
References
Agetsuma, N. (1995). Foraging strategies of Yakushima macaques (Macaca fuscata yakui). International Journal of Primatology, 16(4), 595–609.
Agostini, I. (2009). Ecology and behavior of two howler monkey species (Alouatta guariba clamitans and Alouatta caraya) living in sympatry in northeastern Argentina. PhD dissertation, Università degli Studi di Roma “La Sapienza”, Rome, Italy.
Agostini, I., Holzmann, I., & Di Bitetti, M. S. (2010a). Ranging patterns of two syntopic howler monkey species (Alouatta guariba and A. caraya) in Northeastern Argentina. International Journal of Primatology, 31, 363–381. https://doi.org/10.1007/s10764-010-9390-x.
Agostini, I., Holzmann, I., & Di Bitetti, M. S. (2010b). Are howler monkey species ecologically equivalent? Trophic niche overlap in syntopic Alouatta guariba clamitans and Alouatta caraya. American Journal of Primatology, 72(2), 173–186. https://doi.org/10.1002/ajp.20775.
Agostini, I., Holzmann, I., & Di Bitetti, M. S. (2012). Influence of seasonality, group size, and presence of a congener on activity patterns of howler monkeys. Journal of Mammalogy, 93(3), 645–657. https://doi.org/10.1644/11-MAMM-A-070.1.
Alba-Mejia, L., Caillaud, D., Montenegro, O. L., Sánchez-Palomino, P., & Crofoot, M. C. (2013). Spatiotemporal interactions among three neighboring groups of free-ranging white-footed tamarins (Saguinus leucopus) in Colombia. International Journal of Primatology, 34(6), 1281–1297. https://doi.org/10.1007/s10764-013-9740-6.
Altmann, J. (1974). Observational study of behaviour: sampling methods. Behaviour, 49, 227–267.
Amato, K. R., Leigh, S. R., Kent, A., Mackie, R. I., Yeoman, C. J., Stumpf, R. M., ... & Garber, P. A. (2015). The gut microbiota appears to compensate for seasonal diet variation in the wild black howler monkey (Alouatta pigra). Microbial Ecology, 69(2), 434-443.
Aristizabal, J. F., Rothman, J. M., García-Fería, L. M., & Serio-Silva, J. C. (2016). Contrasting time-based and weight-based estimates of protein and energy intake of black howler monkeys (Alouatta pigra). American Journal of Primatology, 79(4), e22611. https://doi.org/10.1002/ajp.22611.
Arroyo-Rodríguez, V., & Mandujano, S. (2006). Forest fragmentation modifies habitat quality for Alouatta palliata. International Journal of Primatology, 27(4), 1079–1096.
Back, J. P., & Bicca-Marques, J. C. (2019). Supplemented howler monkeys eat less wild fruits, but do not change their activity budgets. American Journal of Primatology, 81(9), e23051. https://doi.org/10.1002/ajp.23051.
Behie, A. M., & Pavelka, M. S. (2015). Fruit as a key factor in howler monkey population density: conservation implications. In M. M. Kowalewski, P. A. Garber, L. Cortés-Ortiz, B. Urbani, & D. Youlatos (Eds.), Howler monkeys Behavior, Ecology, and Conservation (pp. 357–382). Springer.
Behie, A. M., Pavelka, M. S., & Chapman, C. A. (2010). Sources of variation in fecal cortisol levels in howler monkeys in Belize. American Journal of Primatology, 72(7), 600–606. https://doi.org/10.1002/ajp.20813.
Benítez-Malvido, J., & Martínez-Ramos, M. (2003). Impact of forest fragmentation on understory plant species richness in Amazonia. Conservation Biology, 17(2), 389–400.
Bersacola, E., Svensson, M. S., & Bearder, S. K. (2015). Niche partitioning and environmental factors affecting abundance of strepsirrhines in Angola. American Journal of Primatology, 77(11), 1179–1192. https://doi.org/10.1002/ajp.22457.
Bestley, S., Jonsen, I. D., Hindell, M. A., Harcourt, R. G., & Gales, N. J. (2015). Taking animal tracking to new depths: synthesizing horizontal–vertical movement relationships for four marine predators. Ecology, 96(2), 417–427. https://doi.org/10.1890/14-0469.1
Bicca-Marques, J. C. (2003). How do howler monkeys cope with habitat fragmentation? In M. K. Larsh (Ed.), Primates in Fragments: Ecology and Conservation (pp. 283–303). Springer.
Bicca-Marques, J. C., Chaves, Ó. M., & Hass, G. P. (2020). Howler monkey tolerance to habitat shrinking: Lifetime warranty or death sentence? American Journal of Primatology, 82(4), 1–9. https://doi.org/10.1002/ajp.23089.
Bolt, L. M., Russell, D. G., & Schreier, A. L. (2021). Anthropogenic edges impact howler monkey (Alouatta palliata) feeding behaviour in a Costa Rican rainforest. Primates, 62(4), 647–657.
Bonadonna, G., Zaccagno, M., Torti, V., Valente, D., De Gregorio, C., Randrianarison, R. M., Tan, C., Gamba, M., & Giacoma, C. (2020). Intra-and intergroup spatial dynamics of a pair-living singing primate, Indri indri: a multiannual study of three indri groups in Maromizaha Forest, Madagascar. International Journal of Primatology, 41(2), 224–245. https://doi.org/10.1007/s10764-019-00127-5
Bonvicino, C. R. (1989). Ecologia e comportamento de Alouatta belzebul (Primates: Cebidae) na mata atlântica. Revista Nordestina de Bilogia, 6, 149–179.
Bravo, S. P., & Sallenave, A. (2003). Foraging behavior and activity patterns of Alouatta caraya in the northeastern Argentinean flooded forest. International Journal of Primatology, 24, 825–846. https://doi.org/10.1023/A:1024680806342
Bryson-Morrison, N., Tzanopoulos, J., Matsuzawa, T., & Humle, T. (2017). Activity and habitat use of chimpanzees (Pan troglodytes versus) in the anthropogenic landscape of Bossou, Guinea, West Africa. American Journal of Primatology, 38(2), 282–302.
Buss G, Oklander LI, Bicca Marques JC, Hirano ZB, Chaves OM, Mendes SL et al., (2019). Brown howler monkey: Alouatta guariba Humboldt, 1812. In: Primates in Peril. The world’s 25 most endangered primates 2018-2020.
Caillaud, D., Crofoot, M. C., Scarpino, S. V., Jansen, P. A., Garzon-Lopez, C. X., et al (2010). Modelling the spatial distribution and fruiting pattern of a key tree species in a neotropical forest: methodology and potential applications. PLoS One, 5(11), e15002. https://doi.org/10.1371/journal.pone.0015002.
Carvalho Jr., O., Ferrari, S. F., & Strier, K. B. (2004). Diet of a muriqui group (Brachyteles arachnoides) in continuous primary forest. Primates, 45(3), 201–204. https://doi.org/10.1007/s10329-004-0079-7.
Ceccarelli, E., Negrín, A. R., Coyohua-Fuentes, A., Canales-Espinosa, D., & Dias, P. A. D. (2019). An exploration of the factors influencing the spatial behavior of mantled howler monkeys (Alouatta palliata). International Journal of Primatology, 40(2), 197–213. https://doi.org/10.1007/s10764-018-0075-1.
Chapman, C. A. (1990). Ecological constraints on group size in three species of neotropical primates. Folia Primatologica, 55, 1–9.
Chapman, C. A., Chapman, L. J., Wangham, R., Hunt, K., Gebo, D., & Gardner, L. (1992). Estimators of fruit abundance of tropical trees. Biotropica, 527–531.
Chapman, C. A., Wrangham, R. W., & Chapman, L. J. (1995). Ecological constraints on group size: an analysis of spider monkey and chimpanzee subgroups. Behavioral Ecology and Sociobiology, 36, 59–70.
Chaves, Ó. M., Fernandes, F. A., Oliveira, G. T., & Bicca-Marques, J. C. (2019). Assessing the influence of biotic, abiotic, and social factors on the physiological stress of a large Neotropical primate in Atlantic forest fragments. Science of the Total Environment, 690, 705–716. https://doi.org/10.1016/j.scitotenv.2019.07.033.
Chiarello, A. G. (1992). Dieta, padrão de atividades e área de vida de um grupo de bugios (Alouatta fusca) na Reserva de Santa Genebra, Campinas, SP. Masters' dissertation. Universidade Estadual de Campinas, Campinas, Brazil.
Clutton-Brock, T. H., & Harvey, P. H. (1977). Primate ecology and social organization. Journal of Zoology, 183(1), 1–39. https://doi.org/10.1111/j.1469-7998.1977.tb04171.x.
Clutton-Brock, T. H., Rose, K. E., & Guinness, F. E. (1997). Density–related changes in sexual selection in red deer. Proceedings of the Royal Society B: Biological Sciences, 264(1387), 1509–1516.
Cooke, S. J., Hinch, S. G., Wikelski, M., Andrews, R. D., Kuchel, L. J., Wolcott, T. G., & Butler, P. J. (2004). Biotelemetry: a mechanistic approach to ecology. Trends in Ecology & Evolution, 19(6), 334–343.
Cornick, L. A., & Markowitz, H. (2002). Diurnal vocal patterns of the black howler monkey (Alouatta pigra) at Lamanai, Belize. Journal of Mammalogy, 83(1), 159–166. https://doi.org/10.1644/1545-1542(2002)083<0159:DVPOTB>2.0.CO;2.
Cortés-Ortiz, L., Bermingham, E., Rico, C., Rodrıguez-Luna, E., Sampaio, I., & Ruiz-Garcıa, M. (2003). Molecular systematics and biogeography of the Neotropical monkey genus, Alouatta. Molecular Phylogenetics and Evolution, 26(1), 64–81.
Courbin, N., Fortin, D., Dussault, C., & Courtois, R. (2014). Logging-induced changes in habitat network connectivity shape behavioral interactions in the wolf–caribou–moose system. Ecological Monographs, 84(2), 265–285. https://doi.org/10.1890/12-2118.1
Creel, S., & Creel, N. M. (1995). Communal hunting and pack size in African wild dogs, Lycaon pictus. Animal Behaviour, 50(5), 1325–1339.
Cristóbal-Azkarate, J., Urbani, B., & Asensio, N. (2015). Interactions of howler monkeys with other vertebrates: a review. In M. M. Kowalewski, P. A. Garber, L. Cortés-Ortiz, B. Urbani, & D. Youlatos (Eds.), Howler Monkeys: Behavior, Ecology, and Conservation (pp. 141–164). Springer.
Cunha, R. G. T., & Jalles-Filho, E. (2007). The roaring of southern brown howler monkeys (Alouatta guariba clamitans) as a mechanism of active defence of borders. Folia Primatologica, 78(4), 259–271. https://doi.org/10.1159/000105545.
Cunningham, E., & Janson, C. (2007). Integrating information about location and value of resources by white faced-saki monkeys (Pithecia pithecia). Animal Cognition, 10, 293–304.
Dasilva, G. L. (1992). The western black-and-white colobus as a low-energy strategist: activity budgets, energy expenditure and energy intake. Journal of Animal Ecology, 61, 79–91. https://doi.org/10.2307/5511.
de Cunha, R. G. T., de Oliveira, D. A. G., Holzmann, I., & Kitchen, D. M. (2015). Production of loud and quiet calls in howler monkeys. In M. M. Kowalewski, P. A. Garber, L. Cortés-Ortiz, B. Urbani, & D. Youlatos (Eds.), Howler monkeys: Adaptive radiation, systematics, and morphology (pp. 337–369). Springer.
Di Bitetti, M. S., Di Blanco, Y. E., Pereira, J. A., Paviolo, A., & Pírez, I. J. (2009). Time partitioning favors the coexistence of sympatric crab-eating foxes (Cerdocyon thous) and pampas foxes (Lycalopex gymnocercus). Journal of Mammalogy, 90(2), 479–490.
Di Fiore, A., Link, A., & Campbell, C. J. (2011). The atelines: behavioral and socioecological diversity in a New World monkey radiation. In C. J. Campbell, A. Fuentes, M. C. Mackinnon, S. K. Bearder, & R. M. Stumpf (Eds.), Primates in Perspective (pp. 155–188). Oxford University Press.
Dias, L. G., & Strier, K. B. (2000). Agonistic encounters between muriquis, Brachyteles arachnoides hypoxanthus (Primates, Cebidae), and other animals at the Estação Biológica de Caratinga, Minas Gerais, Brazil. Neotropical Primates, 8, 138–141.
dos Santos-Barnett, T. C., Cavalcante, T., Boyle, S. A., Matte, A. L., Bezerra, B. M., de Oliveira, T. G., & Barnett, A. A. (2022). Pulp fiction: why some populations of ripe-fruit specialists Ateles chamek and A. marginatus prefer insect-infested foods. International Journal of Primatology. https://doi.org/10.1007/s10764-022-00284-0.
Falótico, T., Mendonça-Furtado, O., Fogaça, M. D., Tokuda, M., Ottoni, E. B., & Verderane, M. P. (2021). Wild robust capuchin monkey interactions with sympatric primates. Primates, 62, 659–666. https://doi.org/10.1007/s10329-021-00913-x.
Ferreguetti, A. C., Oliveira, A., Pereira, B. C., Santori, R. T., Geise, L., & Bergallo, H. G. (2020). Encounter rate and behavior of Alouatta guariba clamitans in the Ilha Grande State Park, Rio de Janeiro State, Brazil. Zoologia (Curitiba), 37, e36846. https://doi.org/10.3897/zoologia.37.e36846.
Fortes, V. B., Bicca-Marques, J. C., Urbani, B., Fernández, V. A., & Da Silva Pereira, T. (2015). Ranging behavior and spatial cognition of howler monkeys. In M. M. M. Kowalewski, P. A. Garber, L. Cortés-Ortiz, B. Urbani, & D. Youlatos (Eds.), Howler Monkeys: Behavior, Ecology, and Conservation (pp. 219–258). Springer.
Gibson, L., & Koenig, A. (2012). Neighboring groups and habitat edges modulate range use in Phayre’s leaf monkeys (Trachypithecus phayrei crepusculus). Behavioral Ecology and Sociobiology, 66(4), 633–643. https://doi.org/10.1007/s00265-011-1311-2.
Gómez-Posada, C., & Londoño, J. M. (2012). Alouatta seniculus: density, home range and group structure in a bamboo forest fragment in the Colombian Andes. Folia Primatologica, 83(1), 56–65. https://doi.org/10.1159/000339803
González-Solís, J., Guix, J. C., Mateos, E., & Llorens, L. (2001). Population density of primates in a large fragment of the Brazilian Atlantic rainforest. Biodiversity and Conservation, 10(8), 1267–1282. https://doi.org/10.1023/A:1016678126099.
Goodale, E., Beauchamp, G., & Ruxton, G. (2017). Mixed-species groups of animals: behavior, community structure, and conservation. Academic Press.
Gross, M. (2017). Primates in peril. Current Biology, 27(12), 573–576. https://doi.org/10.1016/j.cub.2017.06.002.
Harris, T. R., & Chapman, C. A. (2007). Variation in diet and ranging of black and white colobus monkeys in Kibale National Park, Uganda. Primates, 48(3), 208–221.
Hartig, F. (2017). DHARMa: Residual diagnostics for hierarchical (multi-level/mixed) regression models. https://cran.r-project.org/package=DHARMa. Accessed Mar 2023
Heymann, E. W., & Buchanan-Smith, H. M. (2000). The behavioural ecology of mixed-species troops of callitrichine primates. Biological Reviews, 75(2), 169–190.
Hladik, C. M. (1978). Adaptive strategies of primates in relation to leaf eating. In G. G. Montgomery (Ed.), The Ecology of arboreal Folivores (pp. 373–395). Smithsonian Institution Press.
Hobson, K. A., Norris, D. R., Kardynal, K. J., & Yohannes, E. (2019). Animal migration: a context for using new techniques and approaches. In Tracking animal migration with stable isotopes (pp. 1–23). Academic Press.
Houle, A., Chapman, C. A., & Vickery, W. L. (2010). Intratree vertical variation of fruit density and the nature of contest competition in frugivores. Behavioral Ecology and Sociobiology, 64(3), 429–441.
Isbell, L. A. (1991). Contest and scramble competition: patterns of female aggression and ranging behavior among primates. Behavioral Ecology, 2(2), 143–155. https://doi.org/10.1093/beheco/2.2.143.
Izar, P., Verderane, M. P., Peternelli-Dos Santos, L., Mendonça-Furtado, O., Presotto, A., Tokuda, M., et al (2012). Flexible and conservative features of social systems in tufted capuchin monkeys: comparing the socioecology of Sapajus libidinosus and Sapajus nigritus. American Journal of Primatology, 74(4), 315–331. https://doi.org/10.1002/ajp.20968.
Janmaat, K. R., de Guinea, M., Collet, J., Byrne, R. W., Robira, B., van Loon, E., Jang, H., Biro, D., Ramos-Fernandez, G., Ross, C., Presotto, A., Allritz, M., Alavi, S., & van Belle, S. (2021). Using natural travel paths to infer and compare primate cognition in the wild. Iscience, 24(4), 102343.
Janson, C. (1985). Aggressive competition and individual food consumption in wild brown capuchin monkeys (Cebus apella). Behavioral Ecology and Sociobiology, 18(2), 125–138.
Janson, C. H., & Van Schaik, C. P. (1988). Recognizing the many faces of primate food competition: methods. Behaviour, 105(1/2), 165–186.
Jerusalinsky, L., Bicca-Marques, J.C., Neves, L.G., Alves, S.L., Ingberman, B., Buss, G., Fries, B.G., Alonso, A.C., da Cunha, R.G.T., Miranda, J.M.D., Talebi, M., de Melo, F.R., Mittermeier, R.A. & Cortes-Ortíz, L. (2021). Alouatta guariba (amended version of 2020 assessment). The IUCN Red List of Threatened Species. https://doi.org/10.2305/IUCN.UK.2021-1.RLTS.T39916A190417874.en
Jung, L., Mourthe, I., Grelle, C. E., Strier, K. B., & Boubli, J. P. (2015). Effects of local habitat variation on the behavioral ecology of two sympatric groups of brown howler monkey (Alouatta clamitans). PLoS One, 10(7), e0129789. https://doi.org/10.1371/journal.pone.0129789.
Kamilar, J. M., & Beaudrot, L. (2013). Understanding primate communities: Recent developments and future directions. Evolutionary Anthropology, 22(4), 174–185. https://doi.org/10.1002/evan.21361.
Kernohan, B. J., Gitzen, R. A., & Millspaugh, J. J. (2001). Analysis of animal space use and movements. In J. J. Millspaugh & J. M. Marzluff (Eds.), Radio tracking and animal populations (pp. 125–166). Academic Press.
Kitchen, D. W., Cunha, R. G. T., Holzmann, I., & Oliveira, D. A. G. (2015). Function of loud calls in howler monkeys. In M. M. Kowalewski, P. A. Garber, L. Cortés-Ortiz, B. Urbani, & D. Youlatos (Eds.), Howler monkeys: adaptive radiation, systematics, and morphology (pp. 369–402). Springer.
Knowlton, J. L., & Graham, C. H. (2010). Using behavioral landscape ecology to predict species’ responses to land-use and climate change. Biological Conservation, 143(6), 1342–1354. https://doi.org/10.1016/j.biocon.2010.03.011.
Kowalewski, M. M. (2007). Patterns of affiliation and co-operation in howler monkeys: an alternative model to explain social organization in non-human primates. PhD dissertation, University of Illinois, Urbana, USA.
Kurihara, Y., & Muto, H. (2021). Behavioral responses of Japanese macaques to playback-simulated intergroup encounters. Behavioural Processes, 182, 104279.
Laundré, J. W., Hernández, L., & Ripple, W. J. (2010). The landscape of fear: ecological implications of being afraid. The Open Ecology Journal, 3, 1–7.
Markham, A. C., Alberts, S. C., & Altmann, J. (2012). Intergroup conflict: ecological predictors of winning and consequences of defeat in a wild primate population. Animal Behaviour, 84(2), 399–403. https://doi.org/10.1016/j.anbehav.2012.05.009.
Martins, M. M. (2008). Fruit diet of Alouatta guariba and Brachyteles arachnoides in Southeastern Brazil: comparison of fruit type, color, and seed size. Primates, 49(1), 1–8.
Matsuda, I., Clauss, M., Tuuga, A., Sugau, J., Hanya, G., Yumoto, T., ... & Hummel, J. (2017). Factors affecting leaf selection by foregut-fermenting proboscis monkeys: new insight from in vitro digestibility and toughness of leaves. Scientific Reports, 7(1), 1-10.
Mendes, S. L. (1989). Estudo ecológico de Alouatta fusca (Primates: Cebidae) na Estação Biológica de Caratinga, MG. Revista Nordestina de Biologia, 71–104.
Milton, K. (1979). Factors influencing leaf choice by howler monkeys: a test of some hypotheses of food selection by generalist herbivores. The American Naturalist, 114(3), 362–378.
Milton, K. (1980). The foraging strategy of howler monkeys: a study in primate economics. Columbia University Press.
Milton, K. (1998). Physiological ecology of howlers (Alouatta): energetic and digestive considerations and comparison with the Colobine. International Journal of Primatology, 19, 513–548. https://doi.org/10.1023/A:1020364523213.
Miranda, J., & Passos, F. C. (2004). Hábito alimentar de Alouatta guariba (Humboldt)(Primates, Atelidae) em Floresta de Araucária, Paraná, Brasil. Revista Brasileira de Zoologia, 21(4), 821–826.
Morellato, L. P. C., Talora, D. C., Takahasi, A., Bencke, C. C., Romera, E. C., & Zipparro, V. B. (2000). Phenology of Atlantic rain forest trees: a comparative study. Biotropica, 32(4b), 811–823. https://doi.org/10.1111/j.1744-7429.2000.tb00620.x.
Müller, C. A., & Manser, M. B. (2007). ‘Nasty neighbours’ rather than ‘dear enemies’ in a social carnivore. Proceedings of the Royal Society B: Biological Sciences, 274(1612), 959–965. https://doi.org/10.1098/rspb.2006.0222.
Nagy, K. A., & Milton, K. (1979). Energy metabolism and food consumption by wild howler monkeys (Alouatta palliata). Ecology, 60(3), 475–480.
Nathan, R., et al (2022). Big-data approaches lead to an increased understanding of the ecology of animal movement. Science, 375(6582), eabg1780.
Oklander L. I., Buss G., Bicca-Marques J.C., Hirano Z. B., Chaves O. M., Jardim M. M. A., Valença-Montenegro M. M., Mendes S. L., Neves L. G., Kowalewski M., Melo F. R., Rylands A. B., Jerusalinsky L. (2022). Brown howler monkey: Alouatta guariba Humboldt, 1812. In: Primates in Peril. The world’s 25 most endangered primates 2020-2022.
Pinto, L. P., Barnett, A. A., Bezerra, B. M., Boubli, J.-P., Bowler, M., Cardoso, N., Castelli, C., Rodriguez, M. J. O., Santos, R. R., Setz, E. Z., & Veiga, L. M. (2013). Why we know so little: the challenges of fieldwork on the pitheciines. In L. Veiga, A. Barnett, S. Ferrari, & M. Norconk (Eds.), Evolutionary Biology and Conservation of Titis, Sakis and Uacaris (pp. 145–150). Cambridge University Press.
Pisciotta, K. (2002). The Paranapiacaba forest fragment. In E. Mateos, J. C. Guix, A. Serra, & K. Pisciotta (Eds.), Censuses of vertebrates in a Brazilian Atlantic rainforest area: The Paranapiacaba fragment. University of Barcelona.
Plano de Manejo. (2015). Plano de Manejo Parque Estadual Carlos Botelho. Unidades de Terreno do Parque. Available in <http://observatorio.wwf.org.br/site_media/upload/gestao/planoManejo/PM_PECarlosBotelho_parte_002.pdf>. Accessed: August 2021.
Powell, R. A. (2000). Animal home ranges and territories and home range estimators. In L. Boitoni & T. K. Fuller (Eds.), Research techniques in animal ecology: controversies and consequences (pp. 65–110). Columbia University Press.
Prates, H. M., & Bicca-Marques, J. C. (2008). Age-sex analysis of activity budget, diet, and positional behavior in Alouatta caraya in an orchard forest. International Journal of Primatology, 29, 703–715. https://doi.org/10.1007/s10764-008-9257-6
Pruetz, J. D., & Isbell, L. A. (2000). Correlations of food distribution and patch size with agonistic interactions in female vervets (Chlorocebus aethiops) and patas monkeys (Erythrocebus patas) living in simple habitats. Behavioral Ecology and Sociobiology, 49(1), 38–47.
Prugh, L. R., Sivy, K. J., Mahoney, P. J., Ganz, T. R., Ditmer, M. A., van de Kerk, M., ... & Montgomery, R. A. (2019). Designing studies of predation risk for improved inference in carnivore-ungulate systems. Biological Conservation, 232, 194-207.
Pyke, G. (2019). Animal movements: an optimal foraging approach. In Encyclopedia of animal behavior (pp. 149–156). Elsevier Academic Press.
R Core Team. (2019). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing https://www.R-project.org. Accessed Mar 2023
Raño, M., Kowalewski, M. M., Cerezo, A. M., & Garber, P. A. (2016). Determinants of daily path length in black and gold howler monkeys (Alouatta caraya) in northeastern Argentina. American Journal of Primatology, 78(8), 825–837. https://doi.org/10.1002/ajp.22548.
Reynoso-Cruz, J. E., Rangel-Negrín, A., Coyohua-Fuentes, A., Canales-Espinosa, D., & Dias, P. A. D. (2016). Measures of food intake in mantled howling monkeys. Primates, 57(2), 161–166. https://doi.org/10.1007/s10329-016-0513-7.
Ribeiro, M. C., Metzger, J. P., Martensen, A. C., Ponzoni, F. J., & Hirota, M. M. (2009). The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biological Conservation, 142(6), 1141–1153. https://doi.org/10.1016/j.biocon.2009.02.021.
Ries, L., & Sisk, T. D. (2004). A predictive model of edge effects. Ecology, 85(11), 2917–2926.
Rímoli, J., Nantes, R. D. S., & Júnior, A. L. (2012). Diet and activity patterns of black howler monkeys Alouatta caraya (Humboldt, 1812, Primates, Atelidae) in ecotone Cerrado-Pantanal in the left bank of Aquidauana River, Mato Grosso do Sul, Brazil. Oecologia Australis, 16, 933–948. https://doi.org/10.4257/oeco.2012.1604.15.
Rosa, M. R., Brancalion, P. H., Crouzeilles, R., Tambosi, L. R., Piffer, P. R., Lenti, F. E., ... & Metzger, J. P. (2021). Hidden destruction of older forests threatens Brazil’s Atlantic Forest and challenges restoration programs. Science Advances, 7(4), eabc4547. https://doi.org/10.1126/sciadv.abc4547
Rose, L. M., Perry, S., Panger, M. A., Jack, K., Manson, J. H., Gros-Louis, J., Mackinnon, C. K., & Vogel, E. (2003). Interspecific interactions between Cebus capucinus and other species: data from three Costa Rican sites. International Journal of Primatology, 24(4), 759–796. https://doi.org/10.1023/A:1024624721363
Rosenberger, A. L., & Strier, K. B. (1989). Adaptive radiation of the ateline primates. Journal of Human Evolution, 18(7), 717–750.
Rowley, M. H., & Christian, J. J. (1976). Interspecific aggression between Peromyscus and Microtus females: A possible factor in competitive exclusion. Behavioral Biology, 16(4), 521–525.
Sanchez, G. (2013). PLS Path Modeling with R. Trowchez Editions. Berkeley, 2013. Available in: http://www.gastonsanchez.com/PLS Path Modeling with R.pdf. Accessed October 2022.
Sanz, C. M., Strait, D., Ayina, C. E., Massamba, J. M., Ebombi, T. F., Kialiema, S. N., ... & Morgan, D. B. (2022). Interspecific interactions between sympatric apes. Iscience, 25(10), 105059.
Scarry, C. J. (2013). Between-group contest competition among tufted capuchin monkeys, Sapajus nigritus, and the role of male resource defence. Animal Behavior, 85(5), 931–939. https://doi.org/10.1016/j.anbehav.2013.02.013.
Schoener, T. W. (1974). Resource Partitioning in Ecological Communities: Research on how similar species divide resources helps reveal the natural regulation of species diversity. Science, 185(4145), 27–39.
Schreier, A. L., Bolt, L. M., Russell, D. G., Readyhough, T. S., Jacobson, Z. S., Merrigan-Johnson, C., & Coggeshall, E. M. (2021). Mantled howler monkeys (Alouatta palliata) in a Costa Rican forest fragment do not modify activity budgets or spatial cohesion in response to anthropogenic edges. Folia Primatologica, 92(1), 49–57. https://doi.org/10.1159/000511974.
Sekulic, R. (1982). The function of howling in red howler monkeys (Alouatta seniculus). Behaviour, 38-54. https://doi.org/10.1163/156853982X00517.
Sha, J. C. M., & Hanya, G. (2013). Diet, activity, habitat use, and ranging of two neighboring groups of food-enhanced long-tailed macaques (Macaca fascicularis). American Journal of Primatology, 75(6), 581–592. https://doi.org/10.1002/ajp.22137.
Sih, A. (2005). Predator-prey space use as an emergent outcome of a behavioral response race. Ecology of Predator-Prey Interactions, 256, 78.
Singh, M. R., Singh, M., Ananada Kumar, M., Kumar, H. N., Sharma, A. K., & Sushma, H. S. (2000). Niche separation in sympatric lion-tailed macaques (Macaca silenus) and Nilgiri langur (Presbytis johnii) in an Indian tropical rain forest. Primate Report, 58, 83–95. http://repository.ias.ac.in/89653/1/13p.pdf. Accessed Mar 2023
Snaith, T. V., & Chapman, C. A. (2007). Primate group size and interpreting socioecological models: do folivores really play by different rules? Evolutionary Anthropology, 16(3), 94–106. https://doi.org/10.1002/evan.20132.
Sobral, G., Siqueira Martins, G., & Oliveira, C. A. (2022). Thermal imaging aids behavioural studies: the case of a diurnal neotropical primate. Mastozoologia Neotropical, 29(1), e0622. https://doi.org/10.31687/saremMN.22.29.1.07.e0622.
Sobroza, T. V., Pequeno, P. A. C. L., Gordo, M., Kinapp, N. M., Barnett, A. A., & Spironello, W. (2021). Does co-occurrence drive vertical niche partitioning in parapatric tamarins (Saguinus spp.)? Austral Ecology, 46, 1335–1342. https://doi.org/10.1111/aec.13085.
Strier, K. B. (1987). Ranging behavior of woolly spider monkeys, or muriquis, Brachyteles arachnoides. International Journal of Primatology, 8(6), 575–591.
Strier, K. B. (1989). Effects of patch size on feeding associations in muriquis (Brachyteles arachnoides). Folia Primatologica, 52, 70–77. https://doi.org/10.1159/000156383.
Talebi, M., Bastos, A., & Lee, P. C. (2005). Diet of southern muriquis in continuous Brazilian Atlantic Forest. International Journal of Primatology, 26(5), 1175–1187. https://doi.org/10.1007/s10764-005-6463-3.
Teichroeb, J. A., & Sicotte, P. (2009). Test of the ecological-constraints model on ursine colobus monkeys (Colobus vellerosus) in Ghana. American Journal of Primatology, 71(1), 49–59. https://doi.org/10.1002/ajp.20617.
Temeles, E. J. (1994). The role of neighbours in territorial systems: when are they'dear enemies'? Animal Behavior, 47(2), 339–350. https://doi.org/10.1006/anbe.1994.1047.
Terborgh, J. (2014). Five new world primates. Princeton University Press.
Van Belle, S., & Estrada, A. (2020). The influence of loud calls on intergroup spacing mechanism in black howler monkeys (Alouatta pigra). International Journal of Primatology, 41(2), 265–286.
Van Belle, S., Estrada, A., & Garber, P. A. (2013). Spatial and diurnal distribution of loud calling in black howlers (Alouatta pigra). International Journal of Primatology, 34(6), 1209–1224. https://doi.org/10.1007/s10764-013-9734-4.
Vogel, E. R., & Janson, C. H. (2007). Predicting the frequency of food-related agonism in white-faced capuchin monkeys (Cebus capucinus), using a novel focal-tree method. American Journal of Primatology, 69(5), 533–550. https://doi.org/10.1002/ajp.20368.
Walter, H. (1973). Vegetation of the Earth in Relation to Climate and the Eco-Physiological Conditions. Springer.
Worton, B. J. (1995). A convex hull-based estimator of home-range size. Biometrics, 51, 1206–1215. https://doi.org/10.2307/2533254.
Acknowledgments
A preliminary version of this paper was presented to a committee in partial fulfilment of the requirements for the PhD degree. Hence, we are indebted to committee members J.C. Bicca-Marques, P. Izar, R. G. Ferreira, and A. Araújo for their important suggestions, which greatly increased the quality increase of this paper. We are also thankful to A. Caccavo for producing the home range figure; I. Campell from Stackoverflow for enabling the calculation of DPL using a simple R code. We would also thank L. Wiseman-Jones for her help in proofreading the English language of this paper. Finally, the authors thank the anonymous referees, the Associate Editor and the Editor-in-Chief, for reviews that greatly enhanced the quality of the manuscript. Fieldwork was conducted within the current legal norms in Brazil (Instituto Chico Mendes de Conservação da Biodiversidade License number 55826-1; Secretaria do Meio Ambiente - Fundação Florestal authorisantion number 009.188/2016; SISGEN register number A165E11).
Inclusion and diversity statement
• One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in science.
• One or more of the authors of this paper self-identifies as a member of the LGBTQ+ community.
• While citing references scientifically relevant for this work, we also actively worked to promote gender balance in our reference list.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001 (to GS), and part by FAPESP (#2017/07954-0) (to LFF).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Addisu Mekonnen
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sobral, G., Fuzessy, L.F. & de Oliveira, C.A. The Challenge of Coexistence: Changes in Activity Budget and Ranging Behaviour of Brown Howler Monkeys in Response to the Presence of Conspecifics and Heterospecifics. Int J Primatol 44, 558–580 (2023). https://doi.org/10.1007/s10764-023-00359-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-023-00359-6