Abstract
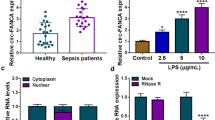
Septic acute kidney injury (AKI) is considered as a severe and common complication of sepsis, with complex pathogenesis. Recently, Circular RNA (circRNA) is considered to be implicated in this disease. This study was intended to elucidate the role of circ_0114428 and the potential mechanism of action in sepsis-induced kidney injury. Sepsis-induced kidney injury cell model was established in human kidney 2 (HK2) cells by the treatment of lipopolysaccharide (LPS). The expression of circ_0114428, CRBN mRNA, and miR-495-3p was detected by quantitative real-time polymerase chain reaction (qRT-PCR). Cell viability was assessed by cell counting kit-8 (CCK-8) assay. The inflammatory response was monitored according to the release of proinflammatory factors by enzyme-linked immunosorbent assay (ELISA). Cell apoptosis was evaluated by flow cytometry assay. The activities of oxidative indicators were examined using the corresponding kits. Endoplasmic reticulum (ER) stress-related proteins and CRBN protein were quantified by western blot. RNA immunoprecipitation (RIP) assay was performed to ensure whether circ_0114428 could interact with Argonaute 2 (Ago2) protein. The potential miRNAs targeted by circ_0114428 were predicted by the bioinformatics tool and screened by RNA pull-down assay. The interaction between miR-495-3p and circ_0114428 or CRBN was validated by dual-luciferase reporter assay. The results showed that circ_0114428 and CRBN were upregulated in septic AKI serum specimens and LPS-induced HK2 cells. Circ_0114428 knockdown attenuated LPS-induced apoptosis, inflammation, oxidative stress, and ER stress, which were rescued by CRBN overexpression. Further analysis revealed that miR-495-3p was targeted by circ_0114428 and directly bound to CRBN, and circ_0114428 regulated CRBN expression by sponging miR-495-3p. Besides, miR-495-3p inhibition also reversed the effects of circ_0114428 knockdown. In conclusion, circ_00114428 knockdown attenuated LPS-induced HK2 cell injury by regulating CRBN expression via targeting miR-495-3p.







Similar content being viewed by others
Data and Materials Availability
All data generated or analyzed during this study are included in this article.
Abbreviations
- AKI:
-
Septic acute kidney injury
- LPS:
-
Lipopolysaccharide
- HK2:
-
Human kidney 2
- ELISA:
-
Enzyme-linked immunosorbent assay
- ER:
-
Endoplasmic reticulum
References
Poston, J.T., and J.L. Koyner. 2019. Sepsis associated acute kidney injury. BMJ 364: k4891.
Alobaidi, R., R.K. Basu, S.L. Goldstein, and S.M. Bagshaw. 2015. Sepsis-associated acute kidney injury. Seminars in Nephrology 35 (1): 2–11.
Joannidis, M., B. Metnitz, P. Bauer, N. Schusterschitz, R. Moreno, W. Druml, and P.G.H. Metnitz. 2009. Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Medicine 35 (10): 1692–1702.
Uchino, S., J.A. Kellum, R. Bellomo, G.S. Doig, H. Morimatsu, S. Morgera, M. Schetz, I. Tan, C. Bouman, E. Macedo, N. Gibney, A. Tolwani, C. Ronco, and Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. 2005. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294 (7): 813–818.
Chen, D., X.Q. Xiong, Y.H. Zang, Y. Tong, B. Zhou, Q. Chen, Y.H. Li, X.Y. Gao, Y.M. Kang, and G.Q. Zhu. 2017. BCL6 attenuates renal inflammation via negative regulation of NLRP3 transcription. Cell Death & Disease 8 (10): e3156.
Esposito, V., F. Grosjean, J. Tan, L. Huang, L. Zhu, J. Chen, H. Xiong, G.E. Striker, and F. Zheng. 2013. CHOP deficiency results in elevated lipopolysaccharide-induced inflammation and kidney injury. American Journal of Physiology. Renal Physiology 304 (4): F440–F450.
Ferre, S., et al. 2019. Renal tubular cell spliced X-box binding protein 1 (Xbp1s) has a unique role in sepsis-induced acute kidney injury and inflammation. Kidney International 96 (6): 1359–1373.
Huang, W., X. Lan, X. Li, D. Wang, Y. Sun, Q. Wang, H. Gao, and K. Yu. 2017. Long non-coding RNA PVT1 promote LPS-induced septic acute kidney injury by regulating TNFalpha and JNK/NF-kappaB pathways in HK-2 cells. International Immunopharmacology 47: 134–140.
Wu, S., et al. 2020. Effects and mechanism of lncRNA CRNDE on sepsis-induced acute kidney injury. Analytical Cellular Pathology (Amsterdam) 2020: 8576234.
Wang, X., et al. 2020. MiR-22-3p suppresses sepsis-induced acute kidney injury by targeting PTEN. Bioscience Reports 40 (6).
Liu, Z., Y. Wang, S. Shu, J. Cai, C. Tang, and Z. Dong. 2019. Non-coding RNAs in kidney injury and repair. American Journal of Physiology. Cell Physiology 317 (2): C177–C188.
Li, C.M., M. Li, Z.C. Ye, J.Y. Huang, Y. Li, Z.Y. Yao, H. Peng, and T.Q. Lou. 2019. Circular RNA expression profiles in cisplatin-induced acute kidney injury in mice. Epigenomics 11 (10): 1191–1207.
Cao, Y., X. Mi, D. Zhang, Z. Wang, Y. Zuo, and W. Tang. 2020. Transcriptome sequencing of circular RNA reveals a novel circular RNA-has_circ_0114427 in the regulation of inflammation in acute kidney injury. Clinical Science (London, England) 134 (2): 139–154.
Xin, W., N. Xiaohua, C. Peilin, C. Xin, S. Yaqiong, and W. Qihan. 2008. Primary function analysis of human mental retardation related gene CRBN. Molecular Biology Reports 35 (2): 251–256.
Yao, C., X. Guo, W.X. Yao, and C. Zhang. 2018. Cereblon (CRBN) deletion reverses streptozotocin induced diabetic osteoporosis in mice. Biochemical and Biophysical Research Communications 496 (3): 967–974.
Mlak, R., A. Szudy-Szczyrek, M. Mazurek, M. Szczyrek, I. Homa-Mlak, M. Mielnik, S. Chocholska, O. Jankowska-Łęcka, T. Małecka-Massalska, and M. Hus. 2019. Polymorphisms in the promotor region of the CRBN gene as a predictive factor for peripheral neuropathy in the course of thalidomide-based chemotherapy in multiple myeloma patients. British Journal of Haematology 186 (5): 695–705.
Yang, H., Z. Song, and D. Hong. 2020. CRBN knockdown mitigates lipopolysaccharide-induced acute lung injury by suppression of oxidative stress and endoplasmic reticulum (ER) stress associated NF-kappaB signaling. Biomedicine & Pharmacotherapy 123: 109761.
Lopez-Girona, A., D. Mendy, T. Ito, K. Miller, A.K. Gandhi, J. Kang, S. Karasawa, G. Carmel, P. Jackson, M. Abbasian, A. Mahmoudi, B. Cathers, E. Rychak, S. Gaidarova, R. Chen, P.H. Schafer, H. Handa, T.O. Daniel, J.F. Evans, and R. Chopra. 2012. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia 26 (11): 2326–2335.
Gil, M., Y.K. Kim, H.Y. kim, H.K. Pak, C.S. Park, and K.J. Lee. 2018. Cereblon deficiency confers resistance against polymicrobial sepsis by the activation of AMP activated protein kinase and heme-oxygenase-1. Biochemical and Biophysical Research Communications 495 (1): 976–981.
Jeck, W.R., J.A. Sorrentino, K. Wang, M.K. Slevin, C.E. Burd, J. Liu, W.F. Marzluff, and N.E. Sharpless. 2013. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 19 (2): 141–157.
Yang, J., M. Huang, L. Zhou, X. He, X. Jiang, Y. Zhang, and G. Xu. 2018. Cereblon suppresses the lipopolysaccharide-induced inflammatory response by promoting the ubiquitination and degradation of c-Jun. The Journal of Biological Chemistry 293 (26): 10141–10157.
Dellinger, R.P., et al. 2013. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Medicine 39 (2): 165–228.
Shi, X., W. Ma, Y. Li, H. Wang, S. Pan, Y. Pan, C. Xu, and L. Li. 2020. CircPRKCI relieves lipopolysaccharide-induced HK2 cell injury by upregulating the expression of miR-545 target gene ZEB2. Biofactors 46: 475–486.
Deng, W., K. Chen, S. Liu, and Y. Wang. 2019. Silencing circular ANRIL protects HK-2 cells from lipopolysaccharide-induced inflammatory injury through up-regulating microRNA-9. Artificial Cells, Nanomedicine, and Biotechnology 47 (1): 3478–3484.
Uddin, M.J., E.S. Pak, and H. Ha. 2018. Carbon monoxide releasing molecule-2 protects mice against acute kidney injury through inhibition of ER stress. The Korean Journal of Physiology and Pharmacology 22 (5): 567–575.
Hesterberg, R.S., M.S. Beatty, Y. Han, M.R. Fernandez, A.A. Akuffo, W.E. Goodheart, C. Yang, S. Chang, C.M. Colin, A.Y. Alontaga, J.M. McDaniel, A.W. Mailloux, J.M.R. Billington, L. Yue, S. Russell, R.J. Gillies, S.Y. Yun, M. Ayaz, N.J. Lawrence, H.R. Lawrence, X.Z. Yu, J. Fu, L.N. Darville, J.M. Koomen, X. Ren, J. Messina, K. Jiang, T.J. Garrett, A.M. Rajadhyaksha, J.L. Cleveland, and P.K. Epling-Burnette. 2020. Cereblon harnesses Myc-dependent bioenergetics and activity of CD8+ T lymphocytes. Blood 136: 857–870.
Lee, S.E., H. Koh, D.J. Joo, B. Nedumaran, H.J. Jeon, C.S. Park, R.A. Harris, and Y.D. Kim. 2020. Induction of SIRT1 by melatonin improves alcohol-mediated oxidative liver injury by disrupting the CRBN-YY1-CYP2E1 signaling pathway. Journal of Pineal Research 68 (3): e12638.
Min, Y., S.M. Wi, J.A. Kang, T. Yang, C.S. Park, S.G. Park, S. Chung, J.H. Shim, E. Chun, and K.Y. Lee. 2016. Cereblon negatively regulates TLR4 signaling through the attenuation of ubiquitination of TRAF6. Cell Death & Disease 7 (7): e2313.
Zhang, J., et al. 2020. miR-495 targets ROCK1 to inhibit lipopolysaccharides-induced WI-38 cells apoptosis and inflammation. The Kaohsiung Journal of Medical Sciences 36 (8): 607–614.
Author information
Authors and Affiliations
Contributions
Yan He designed the study, analyzed the data, and wrote the manuscript. Yuanzhu Sun performed the experiments. Jun Peng analyzed the data.
Corresponding author
Ethics declarations
Ethics Approval
The design of this protocol follows the tenets of the Declaration of Helsinki, approved by the Ethics Committee of People’s Hospital of Rizhao.
Consent for Publication
Written informed consents were obtained from all participants.
Competing Interests
The authors declare no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
He, Y., Sun, Y. & Peng, J. Circ_0114428 Regulates Sepsis-Induced Kidney Injury by Targeting the miR-495-3p/CRBN Axis. Inflammation 44, 1464–1477 (2021). https://doi.org/10.1007/s10753-021-01432-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-021-01432-z