Abstract
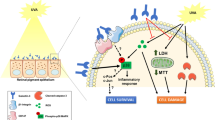
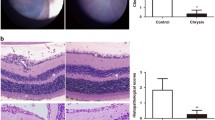
To investigate protective effects of VVN001 on lipopolysaccharide (LPS)-induced inflammatory response in human retinal pigment epithelial (RPE) cells and in a mouse model of endotoxin-induced uveitis (EIU), and to explore the underlying mechanisms. Human primary RPE (hRPE) and ARPE-19 cells were pretreated with or without VVN001 for 1 h followed by 10 μg/mL LPS stimulation for 24 h. mRNA, and protein levels of inflammatory cytokines were analyzed with real-time PCR, western blotting, and ELISA. EIU was induced by intravitreal injection of 125 ng LPS in female BALB/c mice. VVN001 eye drops (1%) were locally administrated every 4 h for 24 h after LPS injection. Clinical scores were assessed with a slit lamp. mRNA and protein levels of inflammatory cytokines were investigated simultaneously. Compared with the LPS group, VVN001 pretreatment significantly reduced mRNA expressions of intercellular adhesion molecule-1 (ICAM-1), IL-6, IL-8, TNF-α, IL-1β, IL-18, caspase-1 in hRPE, and ARPE-19 cells. Protein overproduction of ICAM-1, TNF-α, IL-1β, NLRP3, caspase-1 P20, and p-IκBα/IκBα stimulated by LPS was suppressed by VVN001 pretreatment. In vivo, VVN001 significantly reduced the average clinical score from 5.0 to 1.3 in EIU mice. Furthermore, overproduction of ICAM-1, IL-1β, NLRP3, caspase-1 P20, and p-IκBα/IκBα at mRNA and protein levels were remarkably suppressed by VVN001. VVN001 alleviated the inflammatory response induced by LPS both in vitro and in vivo. The effect of anti-inflammation is associated with inhibiting the overproduction of ICAM-1 and blocking the activation of NLRP3 inflammasome and the NF-κB signaling pathway.








Similar content being viewed by others
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Durrani, O.M., N.N. Tehrani, J.E. Marr, P. Moradi, P. Stavrou, and P.I. Murray. 2004. Degree, duration, and causes of visual loss in uveitis. The British Journal of Ophthalmology 88: 1159–1162.
Siddique, S.S., R. Shah, A.M. Suelves, and C.S. Foster. 2011. Road to remission: a comprehensive review of therapy in uveitis. Expert Opinion on Investigational Drugs 20: 1497–1515.
Merida, S., E. Palacios, A. Navea, and F. Bosch-Morell. 2015. New immunosuppressive therapies in uveitis treatment. International Journal of Molecular Sciences 16: 18778–18795.
Kim, T.W., J.M. Han, Y.K. Han, and H. Chung. 2018. Anti-inflammatory effects of sinomenium acutum extract on endotoxin-induced uveitis in Lewis rats. International Journal of Medical Sciences 15: 758–764.
Yadav, U.C.S., and K.V. Ramana. 2013. Endotoxin-induced uveitis in rodents. Methods in Molecular Biology 1031: 155–162.
Chang, Y.H., C.T. Horng, Y.H. Chen, P.L. Chen, C.L. Chen, C.M. Liang, M.W. Chien, and J.T. Chen. 2008. Inhibitory effects of glucosamine on endotoxin-induced uveitis in Lewis rats. Investigative Ophthalmology & Visual Science 49: 5441–5449.
Holtkamp, G.M., A. K, R. Peek, and A.F. de Vos. 2001. Retinal pigment epithelium-immune system interactions: cytokine production and cytokine-induced changes. Progress in Retinal and Eye Research 20: 29–42.
De Vos, A.F., R. Hoekzema, and A. Kijlstra. 1992. Cytokines and uveitis, a review. Current Eye Research 11: 581–597.
Lawson, C., and S. Wolf. 2009. ICAM-1 signaling in endothelial cells. Pharmacological Reports 61: 22–32.
Perez, V.L., S.C. Pflugfelder, S. Zhang, A. Shojaei, and R. Haque. 2016. Lifitegrast, a novel integrin antagonist for treatment of dry eye disease. The Ocular Surface 14: 207–215.
Martin, C.M., M.S. Lacomba, C.I.S. Molina, R.R. Chamond, J.M.G. Galera, and E.C. Estevez. 2000. Levels of soluble ICAM-1 and soluble IL-2R in the serum and aqueous humor of uveitis patients. Current Eye Research 20: 287–292.
Fu, X., R. Lin, Y. Qiu, Peng Yu, and B. Lei. 2017. Overexpression of angiotensin-converting enzyme 2 ameliorates amyloid beta-induced inflammatory response in human primary retinal pigment epithelium. Investigative Ophthalmology & Visual Science 58: 3018–3028.
Suzuki Y, Ruiz-Ortega M, Lorenzo, O, Ruperez, M, Esteban, V, Egido, J. 2003. Inflammation and angiotensin II. The International Journal of Biochemistry & Cell Biology 35:0–900, 881.
Qiu, Y.G., P.K. Shil, P. Zhu, et al. 2014. Angiotensin-converting enzyme 2 (ACE2) activator diminazene aceturate ameliorates endotoxin-induced uveitis in mice. Investigative Ophthalmology & Visual Science 55: 2809–3818.
Oeckinghaus, A., and S. Ghosh. 2009. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harbor Perspectives in Biology 1: a000034.
Sun, S.C. 2011. Non-canonical NF-kappaB signaling pathway. Cell Research 21: 71–85.
Sato, S., H. Sanjo, K. Takeda, J. Ninomiya-Tsuji, M. Yamamoto, T. Kawai, K. Matsumoto, O. Takeuchi, and S. Akira. 2005. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nature Immunology 6: 1087–1095.
Liu, T., L. Zhang, D. Joo, and S.C. Sun. 2017. NF-kappaB signaling in inflammation. Signal Transduction and Targeted Therapy 2: 17023–17033.
Blenkinsop, T.A., J.S. Saini, A. Maminishkis, K. Bharti, Q. Wan, T. Banzon, M. Lotfi, J. Davis, D. Singh, L.J. Rizzolo, S. Miller, S. Temple, and J.H. Stern. 2015. Human adult retinal pigment epithelial stem cell-derived RPE monolayers exhibit key physiological characteristics of native tissue. Investigative Ophthalmology & Visual Science 56: 7085–7099.
Ablonczy, Z., M. Dahrouj, P.H. Tang, Y. Liu, K. Sambamurti, A.D. Marmorstein, and C.E. Crosson. 2011. Human retinal pigment epithelium cells as functional models for the rpe in vivo. Investigative Ophthalmology & Visual Science 52: 8614–8620.
Soveyd, N., M. Abdolahi, M. Djalali, M. Hatami, A. Tafakhori, P. Sarraf, and N.M. Honarvar. 2018. The combined effects of omega-3 fatty acids and nano-curcumin supplementation on intercellular adhesion molecule-1 (ICAM-1) gene expression and serum levels in migraine patients. CNS & Neurological Disorders Drug Targets 16: 1120–1126.
He, Y., H. Hara, and G. Nunez. 2016. Mechanism and regulation of NLRP3 inflammasome activation. Trends in Biochemical Sciences 41: 1012–1021.
Piccini, A., S. Carta, S. Tassi, D. Lasiglie, G. Fossati, and A. Rubartelli. 2008. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proceedings of the National Academy of Sciences of the United States of America 105: 8067–8072.
Netea, M.G., C.A. Nold-Petry, M.F. Nold, L.A.B. Joosten, B. Opitz, J.H.M. van der Meer, F.L. van de Veerdonk, G. Ferwerda, B. Heinhuis, I. Devesa, C.J. Funk, R.J. Mason, B.J. Kullberg, A. Rubartelli, J.W.M. van der Meer, and C.A. Dinarello. 2009. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood. 113: 2324–2335.
Acknowledgments
We sincerely thank Drs. Wang Shen and Yong Li for providing VVN001 and their technical support.
Funding
This study was financially supported by the National Natural Science Foundation of China grants (81770949) and Henan Key Laboratory of Ophthalmology and Vision Science (CKQ20180099).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qiu, R., Yang, M., Wang, W. et al. The Protective Effects of VVN001 on LPS-Induced Inflammatory Responses in Human RPE Cells and in a Mouse Model of EIU. Inflammation 44, 780–794 (2021). https://doi.org/10.1007/s10753-020-01377-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01377-9