Abstract
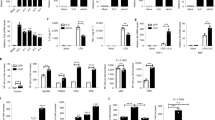
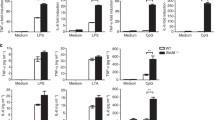
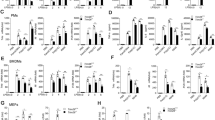
The aims of this study were to investigate the effect of Hsp27 on LPS-induced inflammation and identify the precise mechanisms about how Hsp27 regulates LPS-induced TLR4 signaling in Thp1 cells. Thp1 cells were transfected with Flag-Hsp27 or pcDNA3.1, and then treated with LPS for indicated time. TNF-α, IL-1β, and IL-6 were determined by ELISA. The protein levels of Hsp27, p-Hsp27 (Ser15, Ser78, and Ser82), and TLR4 were measured by Western blotting. In vitro study showed that over-expression of Hsp27 downregulated the release of TNF-α, IL-1β, and IL-6 and suppressed the activation of TLR4 signals after stimulated by LPS. The location of TLR4 and RAB5 was detected by confocal microscopy. Immunoprecipitation was used to determine the ubiquitination and degradation of TLR4 and interaction between Hsp27 and TLR4. Results showed that Hsp27 could promote TLR4 endocytosis and ubiquitination and degradation. Further research revealed that Hsp27 was phosphorylated after LPS, only phosphorylated Hsp27 can interact with TLR4 and inhibit the activation of TLR4 signaling, which was demonstrated by inhibition of Hsp27 phosphorylation with inhibitors or transfection of Hsp27 mutants into Thp1 cells. Phosphorylated Hsp27 reduced the release of TNF-α, IL-1β, and IL-6, and suppressed the activation of TLR4 signaling by promoting TLR4 endocytosis, ubiquitination, and degradation.





Similar content being viewed by others
References
Vatanen, T., A.D. Kostic, E. d'Hennezel, H. Siljander, E.A. Franzosa, M. Yassour, R. Kolde, H. Vlamakis, T.D. Arthur, A.M. Hämäläinen, A. Peet, V. Tillmann, R. Uibo, S. Mokurov, N. Dorshakova, J. Ilonen, S.M. Virtanen, S.J. Szabo, J.A. Porter, H. Lähdesmäki, C. Huttenhower, D. Gevers, T.W. Cullen, M. Knip, DIABIMMUNE Study Group, and R.J. Xavier. 2016. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell 165 (6): 1551. https://doi.org/10.1016/j.cell.2016.05.056.
van der Mark, V.A., M. Ghiboub, C. Marsman, J. Zhao, R. van Dijk, J.K. Hiralall, K.S. Ho-Mok, Z. Castricum, W.J. de Jonge, R.P.J.O. Elferink, and C.C. Paulusma. 2017. Erratum to: Phospholipid flippases attenuate LPS-induced TLR4 signaling by mediating endocytic retrieval of Toll-like receptor 4. Cellular and Molecular Life Sciences 74 (7): 1365. https://doi.org/10.1007/s00018-017-2475-3.
Xie, S., B. Liu, S. Fu, W. Wang, Y. Yin, N. Li, W. Chen, J. Liu, and D. Liu. 2014. GLP-2 suppresses LPS-induced inflammation in macrophages by inhibiting ERK phosphorylation and NF-kappaB activation. Cellular Physiology and Biochemistry 34 (2): 590–602. https://doi.org/10.1159/000363025.
Savant, S.S., S. Sriramkumar, and H.M. O'Hagan. 2018. The role of inflammation and inflammatory mediators in the development, progression, metastasis, and chemoresistance of epithelial ovarian cancer. Cancers (Basel) 10 (8). https://doi.org/10.3390/cancers10080251.
Dandekar, A., Y. Qiu, H. Kim, J. Wang, X. Hou, X. Zhang, Z. Zheng, R. Mendez, F.S. Yu, A. Kumar, D. Fang, F. Sun, and K. Zhang. 2016. Toll-like receptor (TLR) signaling interacts with CREBH to modulate high-density lipoprotein (HDL) in response to bacterial endotoxin. The Journal of Biological Chemistry 291 (44): 23149–23158. https://doi.org/10.1074/jbc.M116.755728.
Garcia-Rodriguez, C., I. Parra-Izquierdo, I. Castanos-Mollor, J. Lopez, J.A. San Roman, and M. Sanchez Crespo. 2018. Toll-like receptors, inflammation, and calcific aortic valve disease. Frontiers in Physiology 9: 201. https://doi.org/10.3389/fphys.2018.00201.
Xie, Z., G. Huang, Z. Wang, S. Luo, P. Zheng, and Z. Zhou. 2018. Epigenetic regulation of Toll-like receptors and its roles in type 1 diabetes. Journal of Molecular Medicine (Berlin, Germany) 96: 741–751. https://doi.org/10.1007/s00109-018-1660-7.
Ding, Y., Y. Qiu, L. Zou, Z. Tan, J. Dai, and W. Xu. 2015. Three conserved MyD88-recruiting TLR residues exert different effects on the human TLR4 signaling pathway. Immunologic Research 62 (2): 213–221. https://doi.org/10.1007/s12026-015-8652-2.
Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nature Reviews. Immunology 4 (7): 499–511. https://doi.org/10.1038/nri1391.
Zhou, S., G. Wang, and W. Zhang. 2018. Effect of TLR4/MyD88 signaling pathway on sepsis-associated acute respiratory distress syndrome in rats, via regulation of macrophage activation and inflammatory response. Experimental and Therapeutic Medicine 15 (4): 3376–3384. https://doi.org/10.3892/etm.2018.5815.
Suh, H.S., M.L. Zhao, N. Choi, T.J. Belbin, C.F. Brosnan, and S.C. Lee. 2009. TLR3 and TLR4 are innate antiviral immune receptors in human microglia: role of IRF3 in modulating antiviral and inflammatory response in the CNS. Virology 392 (2): 246–259. https://doi.org/10.1016/j.virol.2009.07.001.
Yang, Y., H. Zhou, Y. Yang, W. Li, M. Zhou, Z. Zeng, W. Xiong, M. Wu, H. Huang, Y. Zhou, C. Peng, C. Huang, X. Li, and G. Li. 2007. Lipopolysaccharide (LPS) regulates TLR4 signal transduction in nasopharynx epithelial cell line 5-8F via NFkappaB and MAPKs signaling pathways. Molecular Immunology 44 (5): 984–992. https://doi.org/10.1016/j.molimm.2006.03.013.
Lu, Y.C., W.C. Yeh, and P.S. Ohashi. 2008. LPS/TLR4 signal transduction pathway. Cytokine 42 (2): 145–151. https://doi.org/10.1016/j.cyto.2008.01.006.
Beutler, B., X. Du, and A. Poltorak. 2001. Identification of toll-like receptor 4 (Tlr4) as the sole conduit for LPS signal transduction: genetic and evolutionary studies. Journal of Endotoxin Research 7 (4): 277–280.
Wen, L., R.E. Ley, P.Y. Volchkov, P.B. Stranges, L. Avanesyan, A.C. Stonebraker, C. Hu, F.S. Wong, G.L. Szot, J.A. Bluestone, J.I. Gordon, and A.V. Chervonsky. 2008. Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nature 455 (7216): 1109–1113. https://doi.org/10.1038/nature07336.
Cox, D., and H. Ecroyd. 2017. The small heat shock proteins alphaB-crystallin (HSPB5) and Hsp27 (HSPB1) inhibit the intracellular aggregation of alpha-synuclein. Cell Stress & Chaperones 22 (4): 589–600. https://doi.org/10.1007/s12192-017-0785-x.
Li, C., J. Wu, Y. Li, and G. Xing. 2017. Cytoprotective effect of heat shock protein 27 against lipopolysaccharide-induced apoptosis of renal epithelial HK-2 cells. Cellular Physiology and Biochemistry 41 (6): 2211–2220. https://doi.org/10.1159/000475636.
He, K., L. Xia, and J. Zhang. 2018. LPS ameliorates renal ischemia/reperfusion injury via Hsp27 up-regulation. International Urology and Nephrology 50 (3): 571–580. https://doi.org/10.1007/s11255-017-1735-3.
Qi, S., Y. Xin, Z. Qi, Y. Xu, Y. Diao, L. Lan, L. Luo, and Z. Yin. 2014. HSP27 phosphorylation modulates TRAIL-induced activation of Src-Akt/ERK signaling through interaction with beta-arrestin2. Cellular Signalling 26 (3): 594–602. https://doi.org/10.1016/j.cellsig.2013.11.033.
Wu, Y., J. Liu, Z. Zhang, H. Huang, J. Shen, S. Zhang, Y. Jiang, L. Luo, and Z. Yin. 2009. HSP27 regulates IL-1 stimulated IKK activation through interacting with TRAF6 and affecting its ubiquitination. Cellular Signalling 21 (1): 143–150. https://doi.org/10.1016/j.cellsig.2008.10.001.
Oeckinghaus, A., and S. Ghosh. 2009. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harbor Perspectives in Biology 1 (4): a000034. https://doi.org/10.1101/cshperspect.a000034.
Zhang, C., J.T. Jones, H.S. Chand, M.G. Wathelet, C.M. Evans, B. Dickey, J. Xiang, Y.A. Mebratu, and Y. Tesfaigzi. 2018. Noxa/HSP27 complex delays degradation of ubiquitylated IkBalpha in airway epithelial cells to reduce pulmonary inflammation. Mucosal Immunology 11 (3): 741–751. https://doi.org/10.1038/mi.2017.117.
Kim, H.Y., S.Y. Park, and S.Y. Choung. 2018. Enhancing effects of myricetin on the osteogenic differentiation of human periodontal ligament stem cells via BMP-2/Smad and ERK/JNK/p38 mitogen-activated protein kinase signaling pathway. European Journal of Pharmacology 834: 84–91. https://doi.org/10.1016/j.ejphar.2018.07.012.
Chung, S.S., Y. Wu, Q. Okobi, D. Adekoya, M. Atefi, O. Clarke, P. Dutta, and J.V. Vadgama. 2017. Proinflammatory cytokines IL-6 and TNF-alpha increased telomerase activity through NF-kappaB/STAT1/STAT3 activation, and withaferin A inhibited the signaling in colorectal cancer cells. Mediators of Inflammation 2017: 5958429. https://doi.org/10.1155/2017/5958429.
Kamyshova, E.S., M.Y. Shvetsov, I.M. Kutyrina, A.M. Burdennyi, A. Zheng, V.V. Nosikov, and I.N. Bobkova. 2016. Clinical value of TNF, IL-6, and IL-10 gene polymorphic markers in chronic glomerulonephritis. Terapevticheskiĭ Arkhiv 88 (6): 45–50. https://doi.org/10.17116/terarkh201688645-50.
Ghosh, M., J. Subramani, M.M. Rahman, and L.H. Shapiro. 2015. CD13 restricts TLR4 endocytic signal transduction in inflammation. Journal of Immunology 194 (9): 4466–4476. https://doi.org/10.4049/jimmunol.1403133.
Min, Y., S. Lee, M.J. Kim, E. Chun, and K.Y. Lee. 2017. Ubiquitin-specific protease 14 negatively regulates toll-like receptor 4-mediated signaling and autophagy induction by inhibiting ubiquitination of TAK1-binding protein 2 and Beclin 1. Frontiers in Immunology 8: 1827. https://doi.org/10.3389/fimmu.2017.01827.
Gui, J., Y. Huang, and O. Shimmi. 2016. Scribbled optimizes BMP signaling through its receptor internalization to the Rab5 endosome and promote robust epithelial morphogenesis. PLoS Genetics 12 (11): e1006424. https://doi.org/10.1371/journal.pgen.1006424.
Kalin, S., D.P. Buser, and M. Spiess. 2016. A fresh look at the function of Rabaptin5 on endosomes. Small GTPases 7 (1): 34–37. https://doi.org/10.1080/21541248.2016.1140616.
Yamamoto, Y., K. Hosoda, T. Imahori, J. Tanaka, K. Matsuo, T. Nakai, Y. Irino, M. Shinohara, N. Sato, T. Sasayama, K. Tanaka, H. Nagashima, M. Kohta, and E. Kohmura. 2018. Pentose phosphate pathway activation via HSP27 phosphorylation by ATM kinase: a putative endogenous antioxidant defense mechanism during cerebral ischemia-reperfusion. Brain Research 1687: 82–94. https://doi.org/10.1016/j.brainres.2018.03.001.
Vanderwaal, R.P., B. Cha, E.G. Moros, and J.L. Roti Roti. 2006. HSP27 phosphorylation increases after 45 degrees C or 41 degrees C heat shocks but not after non-thermal TDMA or GSM exposures. International Journal of Hyperthermia 22 (6): 507–519. https://doi.org/10.1080/02656730600924406.
Duran, M.C., E. Boeri-Erba, S. Mohammed, J.L. Martin-Ventura, J. Egido, F. Vivanco, and O.N. Jensen. 2007. Characterization of HSP27 phosphorylation sites in human atherosclerotic plaque secretome. Methods in Molecular Biology 357: 151–163. https://doi.org/10.1385/1-59745-214-9:151.
Tanaka, T., M. Iino, and K. Goto. 2018. Sec6 enhances cell migration and suppresses apoptosis by elevating the phosphorylation of p38 MAPK, MK2, and HSP27. Cellular Signalling 49: 1–16. https://doi.org/10.1016/j.cellsig.2018.04.009.
Wang, W., J. Weng, L. Yu, Q. Huang, Y. Jiang, and X. Guo. 2018. Role of TLR4-p38 MAPK-Hsp27 signal pathway in LPS-induced pulmonary epithelial hyperpermeability. BMC Pulmonary Medicine 18 (1): 178. https://doi.org/10.1186/s12890-018-0735-0.
Park, S., H.J. Shin, M. Shah, H.Y. Cho, M.A. Anwar, A. Achek, H.K. Kwon, B. Lee, T.H. Yoo, and S. Choi. 2017. TLR4/MD2 specific peptides stalled in vivo LPS-induced immune exacerbation. Biomaterials 126: 49–60. https://doi.org/10.1016/j.biomaterials.2017.02.023.
Nagai, Y., S. Akashi, M. Nagafuku, M. Ogata, Y. Iwakura, S. Akira, T. Kitamura, A. Kosugi, M. Kimoto, and K. Miyake. 2002. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nature Immunology 3 (7): 667–672. https://doi.org/10.1038/ni809.
Kaiser, F., N. Donos, B. Henderson, R. Alagarswamy, G. Pelekos, D. Boniface, and L. Nibali. 2018. Association between circulating levels of heat-shock protein 27 and aggressive periodontitis. Cell Stress & Chaperones 23: 847–856. https://doi.org/10.1007/s12192-018-0891-4.
Park, S.W., S.W. Chen, M. Kim, V.D. D'Agati, and H.T. Lee. 2009. Human heat shock protein 27-overexpressing mice are protected against acute kidney injury after hepatic ischemia and reperfusion. American Journal of Physiology. Renal Physiology 297 (4): F885–F894. https://doi.org/10.1152/ajprenal.00317.2009.
Kammanadiminti, S.J., and K. Chadee. 2006. Suppression of NF-kappaB activation by Entamoeba histolytica in intestinal epithelial cells is mediated by heat shock protein 27. The Journal of Biological Chemistry 281 (36): 26112–26120. https://doi.org/10.1074/jbc.M601988200.
Medvedev, A.E., W. Piao, J. Shoenfelt, S.H. Rhee, H. Chen, S. Basu, L.M. Wahl, M.J. Fenton, and S.N. Vogel. 2007. Role of TLR4 tyrosine phosphorylation in signal transduction and endotoxin tolerance. The Journal of Biological Chemistry 282 (22): 16042–16053. https://doi.org/10.1074/jbc.M606781200.
Cao, C., Y. Chai, S. Shou, J. Wang, Y. Huang, and T. Ma. 2018. Toll-like receptor 4 deficiency increases resistance in sepsis-induced immune dysfunction. International Immunopharmacology 54: 169–176. https://doi.org/10.1016/j.intimp.2017.11.006.
Schappe, M.S., and B.N. Desai. 2018. Measurement of TLR4 and CD14 receptor endocytosis using flow cytometry. Bio Protocol 8 (14). https://doi.org/10.21769/BioProtoc.2926.
Ruan, J., Z. Qi, L. Shen, Y. Jiang, Y. Xu, L. Lan, L. Luo, and Z. Yin. 2015. Crosstalk between JNK and NF-kappaB signaling pathways via HSP27 phosphorylation in HepG2 cells. Biochemical and Biophysical Research Communications 456 (1): 122–128. https://doi.org/10.1016/j.bbrc.2014.11.045.
Shen, L., Z. Qi, Y. Zhu, X. Song, C. Xuan, P. Ben, L. Lan, L. Luo, and Z. Yin. 2016. Phosphorylated heat shock protein 27 promotes lipid clearance in hepatic cells through interacting with STAT3 and activating autophagy. Cellular Signalling 28 (8): 1086–1098. https://doi.org/10.1016/j.cellsig.2016.05.008.
Acknowledgments
This work was financially supported by grants from the Natural Science Foundation of China (Nos. 81671565, 81771703, and 81471557) and the Priority Academic Program Development of Jiangsu Higher Education Institution (PADD).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, J., Qi, X., Jiang, B. et al. Phosphorylated Heat Shock Protein 27 Inhibits Lipopolysaccharide-Induced Inflammation in Thp1 Cells by Promoting TLR4 Endocytosis, Ubiquitination, and Degradation. Inflammation 42, 1788–1799 (2019). https://doi.org/10.1007/s10753-019-01041-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-019-01041-x