Abstract
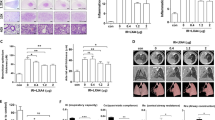
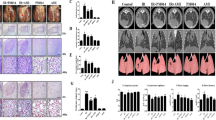
To investigate the effects of aquaporin 4 (AQP4) inhibitor in irradiation-induced pulmonary inflammation in mice. A single dose of 75 Gy was delivered to the left lung of mice to induce radiation pneumonitis. For inhibition of AQP4, 200 mg/kg of TGN-020 was administered i.p. one time per 2 days post-irradiation. Blockade of AQP4 with TGN-020 resulted in the inhibition of inflammatory cell infiltration and the downregulation of inflammatory cytokines (IL-6, IL-17, and TGF-β), chemokines (MIP1a and MCP1), fibrosis-related (Col3al and Fn1), and M2 macrophage marker (Arg1) post-irradiation. Immunofluorescence staining indicated that there was significant fewer M2 macrophage infiltration in the irradiated lung tissues from mice treated with TGN-020. Additionally, depletion of macrophages with liposome clodronate resulted in alleviated lung injury induced by irradiation. Furthermore, adoptive transfer of M1 or M2 macrophages into clodronate-treated mice was performed. The results showed that the administration of M2 macrophages fully reversed the clodronate-induced beneficial effect on inflammation score, thickness, and fibrosis. However, transfer of M1 macrophages only impacted the inflammation score and thickness and did not affect lung fibrosis. AQP4 blockade alleviated the development and severity of irradiated lung damage. This was associated with attenuated infiltration of inflammatory cell, decreased production of pro-inflammatory cytokines, and inhibited activation of M2 macrophages.






Similar content being viewed by others
References
Lancet, T. 2013. Lung cancer: A global scourge. Lancet 382: 659.
Onishi, H., and T. Araki. 2013. Stereotactic body radiation therapy for stage I non-small-cell lung cancer: A historical overview of clinical studies. Japanese Journal of Clinical Oncology 43: 345–350.
Simone, C.B., 2nd, B. Wildt, A.R. Haas, G. Pope, R. Rengan, and S.M. Hahn. 2013. Stereotactic body radiation therapy for lung cancer. Chest 143: 1784–1790.
Ehta, V.I.M. 2005. Radiation pneumonitis and pulmonary fibrosis in non-small-cell lung cancer: Pulmonary function, prediction, and prevention. International Journal of Radiation Oncology, Biology, Physics 63: 5–24.
Yarnold, J., and M.C. Vozenin Brotons. 2010. Pathogenetic mechanisms in radiation fibrosis. Radiother. Oncol. 97: 149–161.
Atsuya, T., T. Yuichiro, and S. Naoko. 2018. Clarithromycin mitigates radiation pneumonitis in patients with lung cancer treated with stereotactic body radiotherapy. J Thorac Dis. 10 (1): 247–261.
Tsoutsou, P.G., and M.I. Koukourakis. 2006. Radiation pneumonitis and fibrosis: Mechanisms underlying its pathogenesis and implications for future research. International Journal of Radiation Oncology, Biology, Physics 66: 1281–1293.
Schaue, D., and W.H. McBride. 2010. Links between innate immunity and normal tissue radiobiology. Radiation Research 173: 406–417.
Marks, L.B., X. Yu, Z. Vujaskovic, W. Small Jr., R. Folz, and M.S. Anscher. 2003. Radiation-induced lung injury. Seminars in Radiation Oncology 13: 333–345.
Demaria, S., E. Pikarsky, M. Karin, L.M. Coussens, Y.C. Chen, E.M. El-Omar, G. Trinchieri, S.M. Dubinett, J.T. Mao, E. Szabo, et al. 2010. Cancer and inflammation: Promise for biologic therapy. Journal of Immunotherapy 33: 335–351.
Sorani, M.D. 2008. Novel variants in human aquaporin-4 reduce cellular water permeability. Human Molecular Genetics 17 (15): 2379–2389.
Verkman, A.S. 2013. Biology of AQP4 and anti-AQP4 antibody: Therapeutic implications. Brain Pathol 23 (6): 684–695.
Sun, C.Y., Y.X. Zhao, and W. Zhong. 2014. The expression of aquaporins 1 and 5 in rat lung after thoracic irradiation. Journal of Radiation Research 55 (4): 683–689.
Bloch, O., and G.T. Manley. 2007. The role of aquaporin-4 in cerebral water transport and edema. Neurosurg. Focus. 22: E3.
Xu, M., W. Su, and Q.P. Xu. 2010. Aquaporin-4 and traumatic brain edema. Chinese Journal of Traumatology 13 (2): 103–110.
Liu, S., J. Mao, T. Wang, and X. Fu. 2017. Downregulation of aquaporin-4 protects brain against hypoxia ischemia via anti-inflammatory mechanism. Molecular Neurobiology 54 (8): 6426–6435.
Ayasoufi, K., N. Kohei, M. Nicosia, et al. 2018. Aquaporin 4 blockade improves survival of murine heart allografts subjected to prolonged cold ischemia. American Journal of Transplantation 00: 1–9.
Kim, M.-G., S.C. Kim, Y.S. Ko, H.Y. Lee, S.-K. Jo, and W. Cho. 2015. The role of M2 macrophages in the progression of chronic kidney disease following acute kidney injury. PLoS One 10 (12): e0143961.
Li, Q., G. Eades, Y. Yao, Y. Zang, and Q. Zhou. 2014. Characterization of a stem-like subpopulation in basal-like ductal carcinoma in situ (DCIS) lessons. The Journal of Biological Chemistry 289 (3): 1303–1312.
McCloy, R.A., S. Rogers, C.E. Caldon, T. Lorca, A. Castro, and A. Burgess. 2014. Partial inhibition of Cdk1 in G 2 phase overrides the SAC and decouples mitotic events. Cell Cycle 13 (9): 1400–1412.
Kim, J.Y., D. Shin, Lee Gihyun, J.M. Kim, and D.W. Kim. 2017. Standardized herbal formula PM014 inhibits radiation-induced pulmonary inflammation in mice. Sci Rep 7: 45001.
Hong, Z.Y., S.H. Eun, K. Park, W.H. Choi, J.I. Lee, E.J. Lee, J.M. Lee, M.D. Story, and J. Cho. 2014. Development of a small animal model to simulate clinical stereotactic body radiotherapy-induced central and peripheral lung injuries. Journal of Radiation Research 55: 648–657.
Dabjan, M.B., C.M. Buck, and I.L. Jackson. 2016. A survey of changing trends in modelling radiation lung injury in mice: Bringing out the good, the bad, and the uncertain. Laboratory Investigation 96 (9): 936–949.
Alnajar, A., C. Nordhoff, T. Schied, R. Chiquet-Ehrismann, K. Loser, T. Vogl, S. Ludwig, and V. Wixler. 2013. The LIM-only protein FHL2 attenuates lung inflammation during bleomycin-induced fibrosis. PLoS One 8 (11): e81356.
Kakugawa, T., et al. 2004. Pirfenidone attenuates expression of HSP47 in murine bleomycin-induced pulmonary fibrosis. The European Respiratory Journal 1: 57–65.
Sempowski, G.D., M.P. Beckmann, S. Derdak, and R.P. Phipps. 1994. Subsets of murine lung fibroblasts express membrane-bound and soluble IL-4 receptors. Role of IL-4 in enhancing fibroblast proliferation and collagen synthesis. J. Immunol. 152: 3606–3614.
Wu, Z., et al. 2013. Effects of carbon ion beam irradiation on lung injury and pulmonary fibrosis in mice. Experimental and Therapeutic Medicine 5: 771–776.
Wolf, J., S. Rose-John, and C. Garbers. 2014. Interleukin-6 and its receptors: A highly regulated and dynamic system. Cytokine 70: 11–20.
Scheller, J., A. Chalaris, D. Schmidt-Arras, and S. Rose-John. 2011. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochimica et Biophysica Acta 1813 (5): 878–888.
Chen, Y., J. Williams, I. Ding, E. Hernady, W. Liu, T. Smudzin, et al. 2002. Radiation pneumonitis and early circulatory cytokine markers. Seminars in Radiation Oncology 12 (1 Suppl 1): 22–33.
Kolls, J.K., and A. Linden. 2004. Interleukin-17 family members and inflammation. Immunity 21 (4): 467–476.
Wilson, M.S., S.K. Madala, T.R. Ramalingamet, B.R. Gochuico, I.O. Rosas, A.W. Cheever, et al. 2010. Bleomycin and IL-1β-mediated pulmonary fibrosis is IL-17A dependent. The Journal of Experimental Medicine 207: 535–552.
Fosslien, E. 2008. Cancer morphogenesis: Role of mitochondrial failure. Annals of Clinical and Laboratory Science 38 (4): 307–329.
Zhang, X.J., J.G. Sun, J. Sun, H. Ming, X.X. Wang, L. Wu, and Z.T. Chen. 2012. Prediction of radiation pneumonitis in lung cancer patients: A systematic review. Journal of Cancer Research and Clinical Oncology 138 (12): 2103–2116.
Johnston, C.J., J.P. Williams, P. Okunieff, and J.N. Finkelstein. 2002. Radiation-induced pulmonary fibrosis: Examination of chemokine and chemokine receptor families. Radiation Research 157: 256–265.
Wermuth, P.J., and S.A. Jimenez. 2015. The significance of macrophage polarization subtypes for animal models of tissue fibrosis and human fibrotic diseases. Clinical and Translational Medicine 4: 2.
Mosmann, T.R., H. Cherwinski, M.W. Bond, M.A. Giedlin, and R.L. Coffman. 2005. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. 1986. Journal of Immunology 175: 5–14.
Funding
This work was supported by the Health and Family Planning Commission of Hubei Province of China (WJ2015CA002) and Scientific Research Project of Wuhan Health and Family Planning Commission (WX15D65).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The data and maternal were available to the corresponding author.
Conflict of Interests
The authors declare that there is no conflict of interests.
Rights and permissions
About this article
Cite this article
Li, Y., Lu, H., Lv, X. et al. Blockade of Aquaporin 4 Inhibits Irradiation-Induced Pulmonary Inflammation and Modulates Macrophage Polarization in Mice. Inflammation 41, 2196–2205 (2018). https://doi.org/10.1007/s10753-018-0862-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-018-0862-z