Abstract
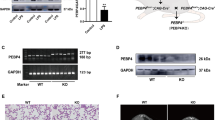
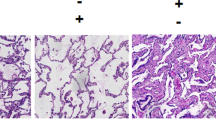
It had been demonstrated that apolipoprotein M (apoM) is an important carrier of sphingosine-1-phosphate (S1P) in blood, and the S1P has critical roles in the pathogenesis of sepsis-induced acute lung injury (ALI). In the present study, we investigated whether apoM has beneficial effects in a mouse model after lipopolysaccharide (LPS)-induced ALI. Forty-eight mice were divided into two groups: male C57BL/6 wild-type (apoM+/+) group (n = 24) and apoM gene-deficient (apoM−/−) group (n = 24) and then randomly subdivided into four subgroups (n = 6 each) according to different intraperitoneal (i.p.) injection: control group, W146 group, LPS group, and LPS + W146 group. Serum levels of interleukin-1 beta (IL-1β) and mRNA levels of IL-1β, interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α), lung histology, wet/dry weight ratio, and immunohistochemistry were measured at 3 h after the baseline and compared in each group. Our results clearly demonstrated that IL-1β mRNA levels and other inflammatory biomarkers were significantly increased in the lungs of LPS-induced ALI apoM−/− mice compared to those of the apoM+/+ mice. Moreover, when apoM+/+ mice were treated with W146, a S1P receptor (S1PR1) antagonist, these inflammatory biomarkers could be significantly upregulated by LPS-induced ALI. Therefore, it suggests that apoM-S1P-S1PR1 signaling might underlie the pathogenesis of ALI and apoM could have physiological benefits to alleviate LPS-induced ALI.





Similar content being viewed by others
References
Dellinger, R.P., M.M. Levy, A. Rhodes, D. Annane, H. Gerlach, S.M. Opal, J.E. Sevransky, C.L. Sprung, I.S. Douglas, R. Jaeschke, T.M. Osborn, M.E. Nunnally, S.R. Townsend, K. Reinhart, R.M. Kleinpell, D.C. Angus, C.S. Deutschman, F.R. Machado, G.D. Rubenfeld, S. Webb, R.J. Beale, J.L. Vincent, and R. Moreno. 2013. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Medicine 39 (2): 165–228.
Chenaud, C., P.G. Merlani, P. Roux-Lombard, D. Burger, S. Harbarth, S. Luyasu, J.D. Graf, J.M. Dayer, and B. Ricou. 2004. Low apolipoprotein A-I level at intensive care unit admission and systemic inflammatory response syndrome exacerbation. Critical Care Medicine 32 (3): 632–637.
Vermont, C.L., M. den Brinker, N. Kakeci, E.D. de Kleijn, Y.B. de Rijke, K.F. Joosten, R. de Groot, and J.A. Hazelzet. 2005. Serum lipids and disease severity in children with severe meningococcal sepsis. Critical Care Medicine 33 (7): 1610–1615.
Chien, J.Y., J.S. Jerng, Yu CJ, and P.C. Yang. 2005. Low serum level of high-density lipoprotein cholesterol is a poor prognostic factor for severe sepsis. Critical Care Medicine 33 (8): 1688–1693.
Berbee, J.F., C.C. van der Hoogt, C.J. de Haas, K.P. van Kessel, G.M. Dallinga-Thie, J.A. Romijn, L.M. Havekes, H.J. van Leeuwen, and P.C. Rensen. 2008. Plasma apolipoprotein CI correlates with increased survival in patients with severe sepsis. Intensive Care Medicine 34 (5): 907–911.
Barlage, S., C. Gnewuch, G. Liebisch, Z. Wolf, F.X. Audebert, T. Gluck, D. Frohlich, B.K. Kramer, G. Rothe, and G. Schmitz. 2009. Changes in HDL-associated apolipoproteins relate to mortality in human sepsis and correlate to monocyte and platelet activation. Intensive Care Medicine 35 (11): 1877–1885.
Grion, C.M., L.T. Cardoso, T.F. Perazolo, A.S. Garcia, D.S. Barbosa, H.K. Morimoto, T. Matsuo, and A.J. Carrilho. 2010. Lipoproteins and CETP levels as risk factors for severe sepsis in hospitalized patients. European Journal of Clinical Investigation 40 (4): 330–338.
Cohen, J. 2002. The immunopathogenesis of sepsis. Nature 420 (6917): 885–891.
Hotchkiss, R.S., and I.E. Karl. 2003. The pathophysiology and treatment of sepsis. The New England Journal of Medicine 348 (2): 138–150.
Annane, D., E. Bellissant, and J.M. Cavaillon. 2005. Septic shock. Lancet 365 (9453): 63–78.
Dellinger, R.P., M.M. Levy, J.M. Carlet, J. Bion, M.M. Parker, R. Jaeschke, K. Reinhart, D.C. Angus, C. Brun-Buisson, R. Beale, T. Calandra, J.F. Dhainaut, H. Gerlach, M. Harvey, J.J. Marini, J. Marshall, M. Ranieri, G. Ramsay, J. Sevransky, B.T. Thompson, S. Townsend, J.S. Vender, J.L. Zimmerman, and J.L. Vincent. 2008. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Medicine 34 (1): 17–60.
Roy, S.K., D. Kendrick, B.D. Sadowitz, L. Gatto, K. Snyder, J.M. Satalin, L.M. Golub, and G. Nieman. 2011. Jack of all trades: pleiotropy and the application of chemically modified tetracycline-3 in sepsis and the acute respiratory distress syndrome (ARDS). Pharmacological Research 64 (6): 580–589.
Parikh, S.M. 2013. Dysregulation of the angiopoietin-Tie-2 axis in sepsis and ARDS. Virulence 4 (6): 517–524.
Ranieri, V.M., G.D. Rubenfeld, B.T. Thompson, N.D. Ferguson, E. Caldwell, E. Fan, L. Camporota, and A.S. Slutsky. 2012. Acute respiratory distress syndrome: the Berlin Definition. JAMA 307 (23): 2526–2533.
Bernard, G.R., A. Artigas, K.L. Brigham, J. Carlet, K. Falke, L. Hudson, M. Lamy, J.R. Legall, A. Morris, and R. Spragg. 1994. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. American Journal of Respiratory and Critical Care Medicine 149 (3 Pt 1): 818–824.
Ferguson, N.D., A.M. Davis, A.S. Slutsky, and T.E. Stewart. 2005. Development of a clinical definition for acute respiratory distress syndrome using the Delphi technique. Journal of Critical Care 20 (2): 147–154.
Kangelaris, K.N., A. Prakash, K.D. Liu, B. Aouizerat, P.G. Woodruff, D.J. Erle, A. Rogers, E.J. Seeley, J. Chu, T. Liu, T. Osterberg-Deiss, H. Zhuo, M.A. Matthay, and C.S. Calfee. 2015. Increased expression of neutrophil-related genes in patients with early sepsis-induced ARDS. American Journal of Physiology. Lung Cellular and Molecular Physiology 308 (11): L1102–L1113.
Petroni, R.C., P.J. Biselli, T.M. de Lima, M.C. Theobaldo, E.T. Caldini, R.N. Pimentel, H.V. Barbeiro, S.A. Kubo, I.T. Velasco, and F.G. Soriano. 2015. Hypertonic saline (NaCl 7.5%) reduces LPS-induced acute lung injury in rats. Inflammation 38 (6): 2026–2035.
Kuebler, W.M., J. Borges, A. Sckell, G.E. Kuhnle, K. Bergh, K. Messmer, and A.E. Goetz. 2000. Role of L-selectin in leukocyte sequestration in lung capillaries in a rabbit model of endotoxemia. American Journal of Respiratory and Critical Care Medicine 161 (1): 36–43.
Ware, L.B., and M.A. Matthay. 2000. The acute respiratory distress syndrome. The New England Journal of Medicine 342 (18): 1334–1349.
Densmore, J.C., P.R. Signorino, J. Ou, O.A. Hatoum, J.J. Rowe, Y. Shi, S. Kaul, D.W. Jones, R.E. Sabina, K.A. Pritchard Jr., K.S. Guice, and K.T. Oldham. 2006. Endothelium-derived microparticles induce endothelial dysfunction and acute lung injury. Shock 26 (5): 464–471.
Wang, X., K.B. Adler, J. Erjefalt, and C. Bai. 2007. Airway epithelial dysfunction in the development of acute lung injury and acute respiratory distress syndrome. Expert Review of Respiratory Medicine 1 (1): 149–155.
Hla, T., M.J. Lee, N. Ancellin, S. Thangada, C.H. Liu, M. Kluk, S.S. Chae, and M.T. Wu. 2000. Sphingosine-1-phosphate signaling via the EDG-1 family of G-protein-coupled receptors. Annals of the New York Academy of Sciences 905: 16–24.
Im, D.S., A.R. Ungar, and K.R. Lynch. 2000. Characterization of a zebrafish (Danio rerio) sphingosine 1-phosphate receptor expressed in the embryonic brain. Biochemical and Biophysical Research Communications 279 (1): 139–143.
Pyne, S., and N. Pyne. 2000. Sphingosine 1-phosphate signalling via the endothelial differentiation gene family of G-protein-coupled receptors. Pharmacology & Therapeutics 88 (2): 115–131.
Anliker, B., and J. Chun. 2004. Cell surface receptors in lysophospholipid signaling. Seminars in Cell & Developmental Biology 15 (5): 457–465.
Rosen, H., and E.J. Goetzl. 2005. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nature Reviews. Immunology 5 (7): 560–570.
Camerer, E., J.B. Regard, I. Cornelissen, Y. Srinivasan, D.N. Duong, D. Palmer, T.H. Pham, J.S. Wong, R. Pappu, and S.R. Coughlin. 2009. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. The Journal of Clinical Investigation 119 (7): 1871–1879.
Christoffersen, C., and L.B. Nielsen. 2013. Apolipoprotein M: bridging HDL and endothelial function. Current Opinion in Lipidology 24 (4): 295–300.
Sammani, S., L. Moreno-Vinasco, T. Mirzapoiazova, P.A. Singleton, E.T. Chiang, C.L. Evenoski, T. Wang, B. Mathew, A. Husain, J. Moitra, X. Sun, L. Nunez, J.R. Jacobson, S.M. Dudek, V. Natarajan, and J.G. Garcia. 2010. Differential effects of sphingosine 1-phosphate receptors on airway and vascular barrier function in the murine lung. American Journal of Respiratory Cell and Molecular Biology 43 (4): 394–402.
Xu, N., and B. Dahlback. 1999. A novel human apolipoprotein (apoM). The Journal of Biological Chemistry 274 (44): 31286–31290.
Feingold, K.R., J.K. Shigenaga, L.G. Chui, A. Moser, W. Khovidhunkit, and C. Grunfeld. 2008. Infection and inflammation decrease apolipoprotein M expression. Atherosclerosis 199 (1): 19–26.
Christoffersen, C., H. Obinata, S.B. Kumaraswamy, S. Galvani, J. Ahnstrom, M. Sevvana, C. Egerer-Sieber, Y.A. Muller, T. Hla, L.B. Nielsen, and B. Dahlback. 2011. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proceedings of the National Academy of Sciences of the United States of America 108 (23): 9613–9618.
Rodriguez, C., M. Gonzalez-Diez, L. Badimon, and J. Martinez-Gonzalez. 2009. Sphingosine-1-phosphate: a bioactive lipid that confers high-density lipoprotein with vasculoprotection mediated by nitric oxide and prostacyclin. Thrombosis and Haemostasis 101 (4): 665–673.
Wang, Z., G. Luo, Y. Feng, L. Zheng, H. Liu, Y. Liang, Z. Liu, P. Shao, M. Berggren-Soderlund, X. Zhang, and N. Xu. 2015. Decreased splenic CD4(+) T-lymphocytes in apolipoprotein M gene deficient mice. BioMed Research International 2015: 293512.
Szarka, R.J., N. Wang, L. Gordon, P.N. Nation, and R.H. Smith. 1997. A murine model of pulmonary damage induced by lipopolysaccharide via intranasal instillation. Journal of Immunological Methods 202 (1): 49–57.
Smith, K.M., J.D. Mrozek, S.C. Simonton, D.R. Bing, P.A. Meyers, J.E. Connett, and M.C. Mammel. 1997. Prolonged partial liquid ventilation using conventional and high-frequency ventilatory techniques: gas exchange and lung pathology in an animal model of respiratory distress syndrome. Critical Care Medicine 25 (11): 1888–1897.
Nahum, A., J. Hoyt, L. Schmitz, J. Moody, R. Shapiro, and J.J. Marini. 1997. Effect of mechanical ventilation strategy on dissemination of intratracheally instilled Escherichia coli in dogs. Critical Care Medicine 25 (10): 1733–1743.
Rotta, A.T., and D.M. Steinhorn. 1998. Partial liquid ventilation reduces pulmonary neutrophil accumulation in an experimental model of systemic endotoxemia and acute lung injury. Critical Care Medicine 26 (10): 1707–1715.
Luo, G., X. Zhang, Q. Mu, L. Chen, L. Zheng, J. Wei, M. Berggren-Soderlund, P. Nilsson-Ehle, and N. Xu. 2010. Expression and localization of apolipoprotein M in human colorectal tissues. Lipids in Health and Disease 9: 102.
Hammes, L.S., R.R. Tekmal, P. Naud, M.I. Edelweiss, N. Kirma, P.T. Valente, K.J. Syrjanen, and J.S. Cunha-Filho. 2008. Up-regulation of VEGF, c-fms and COX-2 expression correlates with severity of cervical cancer precursor (CIN) lesions and invasive disease. Gynecologic Oncology 110 (3): 445–451.
Rubenfeld, G.D., E. Caldwell, E. Peabody, J. Weaver, D.P. Martin, M. Neff, E.J. Stern, and L.D. Hudson. 2005. Incidence and outcomes of acute lung injury. The New England Journal of Medicine 353 (16): 1685–1693.
Steinhauser, M.L., S.L. Kunkel, and C.M. Hogaboam. 1999. New Frontiers in cytokine involvement during experimental sepsis. ILAR Journal 40 (4): 142–150.
Khovidhunkit, W., M.S. Kim, R.A. Memon, J.K. Shigenaga, A.H. Moser, K.R. Feingold, and C. Grunfeld. 2004. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. Journal of Lipid Research 45 (7): 1169–1196.
Chen, H., C. Bai, and X. Wang. 2010. The value of the lipopolysaccharide-induced acute lung injury model in respiratory medicine. Expert Review of Respiratory Medicine 4 (6): 773–783.
Tong, Q., L. Zheng, Q. Kang, O.J. Dodd, J. Langer, B. Li, D. Wang, and D. Li. 2006. Upregulation of hypoxia-induced mitogenic factor in bacterial lipopolysaccharide-induced acute lung injury. FEBS Letters 580 (9): 2207–2215.
Melo, A.C., S.S. Valenca, L.B. Gitirana, J.C. Santos, M.L. Ribeiro, M.N. Machado, C.B. Magalhaes, W.A. Zin, and L.C. Porto. 2013. Redox markers and inflammation are differentially affected by atorvastatin, pravastatin or simvastatin administered before endotoxin-induced acute lung injury. International Immunopharmacology 17 (1): 57–64.
He, Z., X. Chen, S. Wang, and Z. Zou. 2014. Toll-like receptor 4 monoclonal antibody attenuates lipopolysaccharide-induced acute lung injury in mice. Experimental and Therapeutic Medicine 8 (3): 871–876.
Wittwer, T., U.F. Franke, M. Ochs, T. Sandhaus, A. Schuette, S. Richter, N. Dreyer, L. Knudsen, T. Muller, H. Schubert, J. Richter, and T. Wahlers. 2005. Inhalative pre-treatment of donor lungs using the aerosolized prostacyclin analog iloprost ameliorates reperfusion injury. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation 24 (10): 1673–1679.
Katzenstein, A.L., C.M. Bloor, and A.A. Leibow. 1976. Diffuse alveolar damage—the role of oxygen, shock, and related factors. A review. The American Journal of Pathology 85 (1): 209–228.
Thille, A.W., A. Esteban, P. Fernandez-Segoviano, J.M. Rodriguez, J.A. Aramburu, O. Penuelas, I. Cortes-Puch, P. Cardinal-Fernandez, J.A. Lorente, and F. Frutos-Vivar. 2013. Comparison of the berlin definition for acute respiratory distress syndrome with autopsy. American Journal of Respiratory and Critical Care Medicine 187 (7): 761–767.
Zhao, Y., I.A. Gorshkova, E. Berdyshev, D. He, P. Fu, W. Ma, Y. Su, P.V. Usatyuk, S. Pendyala, B. Oskouian, J.D. Saba, J.G. Garcia, and V. Natarajan. 2011. Protection of LPS-induced murine acute lung injury by sphingosine-1-phosphate lyase suppression. American Journal of Respiratory Cell and Molecular Biology 45 (2): 426–435.
Liu, J., P.S. Zhang, Q. Yu, L. Liu, Y. Yang, and H.B. Qiu. 2012. Kinetic and distinct distribution of conventional dendritic cells in the early phase of lipopolysaccharide-induced acute lung injury. Molecular Biology Reports 39 (12): 10421–10431.
Kumaraswamy, S.B., A. Linder, P. Akesson, and B. Dahlback. 2012. Decreased plasma concentrations of apolipoprotein M in sepsis and systemic inflammatory response syndromes. Critical Care 16 (2): R60.
Mihara, Y., T. Miyamoto, Y. Hagari, and M. Mihara. 1997. Rudimentary meningocele of the scalp. The Journal of Dermatology 24 (9): 606–610.
Blaho, V.A., and T. Hla. 2011. Regulation of mammalian physiology, development, and disease by the sphingosine 1-phosphate and lysophosphatidic acid receptors. Chemical Reviews 111 (10): 6299–6320.
Funding
This work was supported by the Program of Bureau of Science and Technology Foundation of Changzhou (CJ20159022), Major Science and Technology Project of Changzhou Municipal Commission of Health and Family Planning (ZD201505), and “333 Project” (BRA2016122) of Jiangsu Province. Dr. Bin Zhu was supported by the Program of Health Top-notch Talent of Changzhou City and Youth Medical Talent of Jiangsu Province.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Animal care was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and was approved by the Animal Use and Protection Committee of Soochow University.
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Zhu, B., Luo, Gh., Feng, Yh. et al. Apolipoprotein M Protects Against Lipopolysaccharide-Induced Acute Lung Injury via Sphingosine-1-Phosphate Signaling. Inflammation 41, 643–653 (2018). https://doi.org/10.1007/s10753-017-0719-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0719-x