Abstract
This study aimed at testing the hypothesis that free-floating plants may facilitate the growth of submerged plants under hypertrophic conditions and intermediate plant density. The effects of Lemna presence on the growth of two submerged plants (Elodea nuttallii and Ceratophyllum demersum) over a nitrogen gradient were experimentally investigated. This was complemented with analysing the presence of C. demersum and E. nuttallii in Hungary and in Germany in relation to the density of free-floating plants. Results showed a negative exponential pattern between underwater light intensity and Lemna cover. Ceratophyllum and Elodea relative growth rate decreased with increasing nitrogen concentrations and additional low Lemna density stimulated Ceratophyllum and suppressed Elodea. Elodea decreased linearly with Lemna density while Ceratophyllum showed a unimodal response. Total algal biomass (epiphytic and planktonic) was higher in Ceratophyllum than in Elodea treatments and decreased rapidly with increasing Lemna density. The field studies showed a positive relationship between Ceratophyllum and a negative one between Elodea and free-floating plant cover. This study clearly showed that free-floating plants can have either facilitating or inhibiting impact on the growth of submerged plants depending on cover density and macrophyte species. The facilitating effect on Ceratophyllum is most likely due to suppressing epiphytic algal growth.
Similar content being viewed by others
Introduction
Light and nutrients are two major abiotic factors that shape abundance patterns of submerged macrophytes together. Besides depth and turbidity, underwater light conditions are also modified by several biotic factors such as shading by neighbouring submerged plants (Szabó et al., 2020), phytoplankton and epiphytic algae (Koleszár et al., 2022b). Also, the presence of grazing snails (Bayley et al., 2007; Yang et al., 2020) and bioturbation by benthic fauna (Adámek & Maršálek, 2013) are important influencers. It is well known that higher availability of nutrients may lead to increased growth of epiphytic algae (Song et al., 2015, 2017), and decreased growth of submerged plants (Yang et al., 2020; Koleszár et al, 2022a). Epiphytic algae not only reduce the light level, but also form a barrier between the macrophyte and the water, thus strongly reducing the availability of nutrients (Sand-Jensen 1977; Roijackers et al., 2004).
It is also known that in small lentic, wind sheltered waterbodies, high nutrient concentration leads to the dominance of free-floating plants (Peeters et al., 2013; Smith et al., 2014; Szabó et al., 2022). These dense mats create dark and anoxic underwater conditions that leave little opportunity for submerged plant to survive (Scheffer et al., 2003). Therefore, due to the reduced light levels in the water body, eutrophication strongly contributes to the decline of submerged vegetation (Phillips et al., 1978, 2016). Shade tolerance and persistence of submerged plants may be the key traits in determining when such a regime shift occurs (Lu et al., 2013). Plants with lower light compensation point or higher shade tolerance can be expected to have higher survival chance in turbid waters or in the shade of floating macrophytes (Szabó et al., 2020; Koleszár et al., 2022a). Free-floating plants reduce available light not only for submerged plants but for phytoplankton and epiphytic algae too. Beyond shading, they also reduce the available nutrients for epiphytes, resulting in their slower growth (Lu et al., 2013; Pinto & O’Farrell, 2014). Consequently, not only submerged plants can be inhibited by the shading effect of free-floating plants, but epiphytic algae as well. Therefore, it can be concluded that the shade of free-floating plants may not always be negative on the growth of submerged plants. It is already well known that intermediate shading stimulates apical elongation, chlorophyll concentration and photosynthetic efficiency of rooted submerged plants (Lu et al., 2013; Szabó et al., 2019, 2020) and decreases root formation and degree of branching (James et al., 2006; Szabó et al., 2019, 2020). However, all of the studies examining the interactions between the two plant groups reported that shading by free-floating plants have obvious negative effect on the biomass-based growth rate of submerged plants (Janes et al., 1996; Morris et al., 2004; Larson 2007).
If the negative impact by epiphytic algae is stronger than shading by free-floating plants, on the other hand, a certain level of floating plant density may in principle favour the growth of submerged macrophytes. Therefore, we hypothesized that under hypertrophic conditions combined with intermediate plant density, the presence of free-floating plants facilitated submerged plant growth due to suppressing algal growth. In the present study, we tested this assumption on two widespread submerged plant species: a rooted [Elodea nuttallii (Planch.) H. St. John] and a rootless one (Ceratophyllum demersum L.), respectively. Here, we evaluated this hypothesis by investigating how free-floating duckweed Lemna gibba L. changed light conditions in the water column and how this affected the growth of algae and the submerged plants C. demersum and E. nuttallii. Furthermore, monitoring data on the occurrence and abundance of C. demersum and E. nuttallii in Hungary and in Germany were studied in relation to the density of free-floating plants.
Materials and methods
Plant collection and preincubation
Fronds of L. gibba (subsequently termed Lemna) and shoots of C. demersum (subsequently termed Ceratophyllum) were collected from Igrice channel (N 47.996376°, E 21.734152°), while shoots of E. nuttallii (subsequently termed Elodea) were obtained from the Eastern Principal Channel, (N 47.860911°, E 21.382270°) NE Hungary. After their collection and before the experiment, we rinsed the plants with tap water removing the epiphytic algae and debris. The plants were preincubated for a month in 10 l plastic boxes containing a general purpose culture medium modified from Barko and Smart (1985). Nitrogen and phosphorus were supplied by adding stock solution of NH4NO3 and K2HPO4 to a final concentration of 2 mg N l−1 and 0.4 mg P l−1, respectively. We added TROPICA supplier micronutrient solution to ensure micronutrient supply using 10,000-fold dilution. The following final concentrations were set in the medium: Fe 0.080, Mn 0.045, Zn 0.002, Cu 0.006 and Mo 0.002 mg l−1, respectively. Illumination was carried out by Philips 400 W metal halogen lamps.
Effects of Lemna density on light attenuation
Underwater light intensities were measured under different Lemna biomass densities in 2 l plastic black covered aquaria (height 11.5 cm, width 11 cm, length 18 cm) containing 2 l culture medium. Initial light intensity was 280 µmol m−2 s−1 photon flux density above the water surface. Sensor of MQ-510 full spectrum quantum metre (Apogee-Instruments, Logan USA) was placed 10 cm below the water surface and photosynthetic active radiation was measured with increasing Lemna biomass (0, 1, 5, 10, 15, 20, 25, 30, 35 and 40 g fresh weight FW) corresponding to 0, 50, 250, 500, 750, 1000, 1250, 1500, 1750 and 2000 g FW m−2. We used the mean of six measurements per biomass density. We repeated the measurement three times using the same Lemna densities detailed above.
Impact of Lemna on submerged plant with nutrient increase
Semi-static conditions were applied to mimic a situation in which nutrient release or replenishment from the sediment to the water column takes place (Szabó et al., 2022). We introduced an initial biomass of 10 ± 0.2 g (500 g m−2) Ceratophyllum (13–15 cm length), and Elodea (13–15 cm length) shoots in 2 l plastic aquaria separately. The sides of the aquaria were covered with black foil to avoid light penetration from the sides. We co-cultured the plants with 0.5 ± 0.02 g FW of Lemna (25 g m−2) resulting 5% of Lemna cover and without Lemna as control. We applied five different nitrogen concentrations to reach 0.5, 1, 2, 5 or 10 mg N l−1 by adding NH4NO3. The initial concentration of phosphorus was the same as in the preincubation. Similarly, we applied the same dilution of microelements that was 1:10,000. Three aquaria per treatment (in total 30–30 aquaria) were incubated for 20 days under 280 µmol m−2 s−1 photon flux density and a photoperiod of 16-h light/8-h dark. The aquaria were kept in a temperature-controlled (24–26°C) water bath. We renewed half of the medium (1 l) twice a week. The medium was drained by gravity and fresh medium was carefully added to the aquaria through a PVC tube placed in the corner in order to avoid any perturbation. At days 12 and 19 (between 6 AM and 4 PM), pH and dissolved oxygen in each aquarium were recorded. At the end of the experiment (day 20), plants were harvested and measured for fresh weight (FW), dried at 80°C for 2 days and then measured again for dry weight (DW). We calculated relative growth rates per day (RGRs) as RGR = (lnFWt – lnFW0)/t in which FWt and FW0 were the fresh weights at time t (20) and time 0, respectively.
Impact of Lemna density on submerged plants
In a subsequent experiment, Ceratophyllum and Elodea shoots (10 ± 0.2 g) were placed separately in 2 l plastic aquaria covered with black plastic foil on their sides. We co-cultured the plants with 0.5, 5, 20, 40 g FW of Lemna (25, 250, 1000, 2000 g m−2) and without Lemna as control. The applied densities resulted zero, low, intermediate, complete and dense cover (0, 5, 50, 200, 400%) on the surface. We applied two different nitrogen concentrations by adding NH4NO3 to reach 2 and 10 mg N l−1. The initial concentration of phosphorus was the same as in the preincubation as well as the dilution factor of microelement solution was 1:10,000. Three aquaria per treatment (in total 30 aquaria) were incubated for 20 days under the same conditions detailed in previous experiment. At the end of the experiment (day 20), epiphytic algae were gently removed from the surface of Ceratophyllum and Elodea shoots and from the side of the aquaria into the media using a paintbrush. After filtration (pore diameter 5–8 µm) of the medium, total algal biomass (epiphytic + planktonic) was dried (80°C for 48 h) and the dry mass was measured. Fresh and dry weights were measured and daily yield of the plants was calculated. At day 19 (4 PM), we analysed water for pH and dissolved oxygen (DO) concentration.
Assessment of macrophyte abundances in field sites
Using the Hungarian Biotic Database of General Directorate of Water Management (OVF, www.ovf.hu/en) and MaPHYTE database (http://data.freshwaterbiodiversity.eu/metadb/bf_mdb_view.php?uid=64da4b8287450&code=22), we compiled macrophyte abundance data from Hungary (2005–2019 June–September), and from Germany (1994–2011 June–September) including the records of either (or both) free-floating plants (FFPs) (Azolla caroliniana Willd., Salvinia natans L., Lemna minor L., L. minuta Kunth, L. gibba L., Spirodela polyrhiza L., Hydrocharis morsus-ranae L.) or submerged species Ceratophyllum demersum/Elodea nuttallii (Birk and Willby 2010). Macrophyte abundance data were converted into mean values of Braun–Blanquet’s cover classes 1–5 (that is, 3%; 15%; 37.5%; 62.5%; 87.5%) using the method of Engloner (2012). To avoid interfering effects by dominance of other submerged plants if Ceratophyllum cover was less than 87.5%, we selected only those sites where total cover of other submerged plants did not exceed the cover of Ceratophyllum or where their total cover was less than 20%. The same filtering method was applied for the selection of Elodea. To avoid disturbance by dominance of rooted floating-leaved plants (i.e. Potamogeton natans L., Nymphaea alba L., Nuphar lutea (L.) Sibth. & Sm., Trapa natans L.) in case total free-floating plant cover was less than 87.5%, we selected only those sites where total cover of floating-leaved plants did not exceed the cover of free-floating plants or where their total cover was less than 20%. Using those data, the abundance of Ceratophyllum or that of Elodea was analysed as a function of free-floating plant abundance (Szabó et al., 2022).
Statistical analyses
For the laboratory experiments, we used Kolmogorov–Smirnov tests to check the normality of the variables. As the measured variables (RGR, yield, biomass of periphytic algae) were normally distributed (P > 0.05), general linear model (GLM) was used to test the significance of the effects of different factors (Lemna density, nitrogen concentration, submerged species) and their interactions on these variables. We checked residuals for normality, and we evaluated homogeneity of variances by Levene's test. We applied Tukey's post hoc tests to evaluate which Lemna density treatments differed from each other. We used pairwise comparisons (PC) to test the variables for significant differences among Lemna treatments, where the mean difference (MD) ± standard error was indicated.
Pearson Chi-square Tests were performed to evaluate whether there was an association between Ceratophyllum and free-floating plants in the Hungarian vegetation survey (n = 992) and between Elodea and free-floating plants in the German dataset (n = 295). All analyses were done using SPSS 16.0 software. Nonlinear models were fitted in Lemna density-dependent light attenuation studies, in density-dependent submerged plant yield, and in field survey. The weighted regression models that considered the standard errors of the means were calculated by LAB Fit curve-fitting software (Silva & Silva, 2018). We choose the best fitting curves based on the simplicity of the function, the lowest confidence intervals and the highest R2 values.
Results
Effects of Lemna density on light attenuation
Underwater light intensity followed a negative exponential pattern with increasing biomass cover. Intermediate Lemna density (48%, 241 g m−2) decreased the underwater light intensity by 50% and complete Lemna density (200%, 1000 g m−2) by 95% (Fig. 1).
Impact of Lemna on submerged plants with nutrient increase
Overall, increasing nitrogen concentration (from 0.5 to 10 mg l−1) significantly decreased the growth rate of the submerged plants (P < 0.001, MD 0.009–0.028 ± 0.002, PC) and Elodea showed significantly higher RGR than Ceratophyllum (P < 0.001, MD 0.052 ± 0.002, PC). The relative growth rate of Ceratophyllum was significantly higher (P < 0.001, MD 0.011 ± 0.002, PC) while RGR of Elodea was significantly lower (P < 0.001, MD 0.017 ± 0.003, PC) with the presence of Lemna comparing to control cultures (Fig. 2a, b).
Impact of Lemna density on submerged plants
Change in plant growth
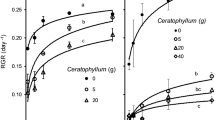
The yield (g m−2 day−1) of Ceratophyllum was significantly stimulated (P < 0.001, MD 8.34 ± 1.67, PC) under intermediate initial Lemna density (50%) compared to control cultures (Fig. 3a, b). Under higher nitrogen concentration (10 mg l−1), the facilitation of Ceratophyllum yield was even higher (P = 0.005, MD 10.57 ± 2.92, PC). However, above this Lemna density level of 50%, further increasing Lemna cover significantly reduced the yield of Ceratophyllum and the highest initial Lemna density (400%) resulted in complete decay of Ceratophyllum (Fig. 3b).
The effect of Lemna density on yield of Ceratophyllum (a, b) and of Elodea (c, d) cultivated in 2 (a, c) and 10 mg N l−1 (b, d) nutrient concentration. Lemna density was measured at the 20th day of the experiment. Means ± SE, n = 3; dotted lines are 95% confidence intervals (c, d). Significant differences (Tukey’s test, P < 0.05) of the yield among treatments are indicated with different lowercase letters; asters indicates significant differences of the Pairwise comparisons (**P < 0.01, ***P < 0.001)
The yield of Elodea grown at 2 mg l−1 N was significantly decreased (P = 0.001, MD 5.83 ± 1.18, PC) under intermediate (50%) or higher initial Lemna density and showed linear pattern with increasing biomass cover. At 10 mg l−1 N, the inhibitory impact of Lemna cover was even stronger where low (5%) initial Lemna density resulted in significantly (P = 0.001, MD 8.03 ± 2.45, PC) reduced Elodea yield. Here, both complete and dense initial density resulted in complete decay of Elodea (Fig. 3c, d). Overall, under intermediate Lemna density or above, the yield of Ceratophyllum was significantly (P < 0.001, MD 12.5 ± 0.87, PC) higher than that of Elodea.
Change in algal growth
Total algal biomass (DW) was significantly higher (P = 0.024, MD 76 ± 31, PC) in the cultures of Ceratophyllum compared to Elodea. Lemna cover strongly reduced algal biomass where even low initial Lemna density (5%) resulted in significantly reduced algal biomass compared to control cultures (Ceratophyllum: P = 0.022, MD 260 ± 96; Elodea: P < 0.001, MD 129 ± 23, PC). Algal biomass values showed negative logarithmic pattern with increasing Lemna biomass both in Ceratophyllum and in Elodea co-cultures (Fig. 4).
The effect of Lemna density on algal biomass cultivated with Ceratophyllum (a) and with Elodea (b) in 10 mg N l−1 nutrient concentration. The indicated Lemna densities were measured at the 20th day of the experiment. Means ± SE, n = 3; dotted lines are 95% confidence intervals. Note There is two times difference in the scale of the two Y-axis in panel a and b
Change in pH and oxygen concentration
Lemna density had significant (P < 0.000, ANOVA) effects on the pH and on the DO of the medium. Both submerged plant species showed a similar pattern with increasing Lemna density (Fig. 5). Regarding to both pH and DO, there were no significant effects between the two species (P = 0.718, ANOVA). In Ceratophyllum and in Elodea cultures, intermediate (50%) or higher initial Lemna density reduced the pH from 9.73 and 9.96 to below 6 (Fig. 5a–c). Complete and dense initial Lemna density reduced DO of the medium below 1.5 mg l−1 in Ceratophyllum cultures and below 0.5 mg l−1 in Elodea cultures.
Field survey
In the Hungarian field survey, high Ceratophyllum coverage was observed significantly more often than expected under high free-floating plant (FFP) cover (> 30%) and less frequent than expected for low FFP (Fig 6a). In addition, low FFP resulted in significantly more observations of low Ceratophyllum abundance (Pearson Chi-Square = 41.304, df = 1, P < 0.001) and Ceratophyllum abundance showed positive linear pattern with increasing FFP cover (Fig 6a).
The Pearson Chi-square test applied to the German survey showed that there were significant differences between the observed and expected frequencies (Pearson Chi-Square = 5.431, df = 1, P = 0.023). The observed frequency of high Elodea cover (> 30% cover) under high FFP cover (> 30% cover) was significantly lower than the expected frequency and the observed frequency of high Elodea under low FFP was significantly higher than the expected frequency. The same analysis showed also that low Elodea occurred significantly more frequently under high FFP and less under low FFP.
We found a negative power function relationship between the cover of FFP and that of Elodea (Fig. 6b). High Elodea density was 4.4 times more likely in those sites where duckweed density was low (0–30% cover) comparing to sites with high duckweed density (cover > 30%).
Discussion
The present study clearly showed that, depending on cover density and species identity, free-floating plants can have either facilitating or inhibiting impact on the growth of submerged plants. The growth responses of the two submerged species (Ceratophyllum, Elodea) to shading by Lemna were completely different. The impact of dense Lemna cover was much more negative on the growth of Elodea compared to Ceratophyllum. Increasing nitrogen concentration also increased the biomass of Lemna that, in turn, reduced both the underwater light availability and algal biomass. Algal growth was strongly reduced even under low (5%) initial Lemna cover. There are several possible reasons for growth stimulation of Ceratophyllum under intermediate Lemna density. Since total algal biomass was nearly two times higher in the presence of Ceratophyllum as compared to Elodea, the strong drop in algal biomass under intermediate Lemna cover could indirectly facilitate the growth of Ceratophyllum (Fig. 7). On the other hand, as there were less algal biomass in the presence of Elodea, an intermediate Lemna cover did not result such a drastic drop in algal biomass. Therefore, shading by Lemna had only an inhibiting impact on Elodea. In the presence of Elodea, reduced algal biomass concurs with the findings that Elodea produces allelopathic substances reducing the growth of several epiphytic and planktonic cyanobacteria and green algal species (Erhard & Gross, 2006; Lürling et al., 2006; Vanderstukken et al., 2014).
It is already well investigated that in water bodies dominated by submerged plants, algal biomass was largely restricted to epiphytic algae, and planktonic algae constituted only a minor fraction of the total algal biomass (Koleszár et al., 2022b). It is also well known that epiphytic algae can reduce light availability for submerged plants by more than 90% (Tóth, 2013). Beyond reducing light, the layer of epiphytic algae on the plant surface limits nutrient availability for the host plants as well. Consequently, there is a possibility that free-floating plants have stimulating effect on submerged plants if the restricting effects on epiphytic algae (i.e. shading + direct contact inhibition) are larger than on free-floating plants.
Field survey of the two submerged plants supported our experimental findings where the abundance of Ceratophyllum correlated positively with the total cover of free-floating plants. On the other hand, there was a strong negative correlation between total cover of free-floating plants and Elodea abundance. Our field survey also indicated that Ceratophyllum tolerated the dense cover of free-floating plants much better than Elodea. Elodea was absent at complete (100%) cover of free-floating plants, while the presence of Ceratophyllum was still 73% in sites with 100% of free-floating plant cover. Based on the basic literature (Szabó et al., 2020, Koleszár et al., 2022a, b), Elodea has considerably low light compensation point comparing to other submerged plants like Myriophyllum spicatum, meaning that Elodea well tolerated the shade. We think that beyond the shading of Lemna, the created anoxia has much more negative effect on Elodea comparing to Ceratophyllum (Morris et al., 2004). The strong resistance of Ceratophyllum to anoxia corresponds well to the field results where dense cover of free-floating vegetation resulted the extinction of all submerged species with exception of Ceratophyllum (Jaklič et al., 2020).
The results of the present experiment on Elodea and FFP support the view that high coverage of free-floating plants may result in a loss of plant species and frequently to complete lack of submerged plants (e.g. Scheffer et al., 2003). The presence of only Ceratophyllum under a FFP layer is a sign that the system may quickly switch to a situation without any submerged plant. Recovery from such a situation might be very difficult since high coverage with FFP may also lead to a deteriorated propagule bank in the sediment (van Zuidam et al., 2012).
Our laboratory experiments may overestimate the facilitating effects of free-floating plants on Ceratophyllum, as a few natural occurring buffering mechanisms were missing in the aquaria. For instance, in field conditions grazing snails consume the epiphytic algae on the surface of Ceratophyllum (Koleszár et al., 2022a). Through top-down control, grazing snails strongly increase the light availability for Ceratophyllum and thus they reduce the negative effect of epiphytic algae (Yang et al., 2020, Koleszár et al., 2022a). Consequently, the grow rate of Lemna free Ceratophyllum cultures would be higher with the presence of snails.
Conclusion
Submerged and free-floating vegetations are regarded as two alternative states that may occur under similar conditions. Here field data implied that there is mutual relationship between Ceratophyllum and FFP. Dense Ceratophyllum mats may support FFP by slowing the water movements and give chance for FFP anchoring on the surface when Ceratophyllum already dominates the water body. On the other hand, under hypertrophic conditions, by reducing the growth of epiphyton, intermediate FFP cover slightly stimulates the growth of Ceratophyllum. Comparing to Elodea, Ceratophyllum tolerates shade and anoxia better; thus, it is a better survivor under denser FFP plant cover. Above a certain nutrient concentration, however, FFP strongly overgrows Ceratophyllum. Exceeding a threshold FFP, plant density eventually causes total decay of Ceratophyllum resulting in the complete dominance of FFP.
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
References
Adámek, Z. & B. Maršalek, 2013. Bioturbation of sediments by benthic macroinvertebrates and fish and its implication for pond ecosystems: a review. Aquac Int 21:1–17. https://doi.org/10.1007/s10499-012-9527-3
Barko, J. W. & R. M. Smart, 1985. Laboratory culture of submerged freshwater macrophytes on natural sediments. Aquatic Botany 21: 251–263. https://doi.org/10.1016/0304-3770(85)90053-1.
Bayley, S., I. Creed, G. Sass & A. Wong, 2007. Frequent regime shifts in trophic states in shallow lakes on the Boreal Plain: alternative “unstable” states? Limnology and Oceanography 52: 2002–2012. https://doi.org/10.4319/lo.2007.52.5.2002.
Engloner, A., 2012. Alternative ways to use and evaluate Kohler’s ordinal scale to assess aquatic macrophyte abundance. Ecological Indicators 20: 238–243. https://doi.org/10.1016/j.ecolind.2012.02.023.
Erhard, D. & E. M. Gross, 2006. Allelopathic activity of Elodea canadensis and Elodea nuttallii against epiphytes and phytoplankton. Aquatic Botany 85: 203–211. https://doi.org/10.1016/j.aquabot.2006.04.002.
Jaklič, M., Š Koren & N. Jogan, 2020. Alien water lettuce (Pistia stratiotes L.) outcompeted native macrophytes and altered the ecological conditions of a Sava oxbow lake (SE Slovenia). Acta Botanica Croatia 79: 35–42. https://doi.org/10.37427/botcro-2020-009.
James, C. S., J. W. Eaton & K. Hardwick, 2006. Responses of three invasive aquatic macrophytes to nutrient enrichment do not explain their observed field displacements. Aquatic Botany 84: 347–353. https://doi.org/10.1016/j.aquabot.2006.01.002.
Janes, R. A., J. W. Eaton & K. Hardwick, 1996. The effects of floating mats of Azolla filiculoides Lam. and Lemna minuta Kunth on the growth of submerged macrophytes. Hydrobiologia 340: 23–26. https://doi.org/10.1007/978-94-011-5782-7_5.
Koleszár, G., B. A. Lukács, P. T. Nagy & S. Szabó, 2022. Shade tolerance as a key trait in invasion success of submerged macrophyte Cabomba caroliniana over Myriophyllum spicatum. Ecology and Evolution 12: e9306. https://doi.org/10.1002/ece3.9306.
Koleszár, G., Z. Nagy, E. T. H. M. Peeters, G. Borics, G. Várbíró, S. Birk & S. Szabó, 2022. The role of epiphytic algae and grazing snails in stable states of submerged and of free-floating plants. Ecosystems 25: 1371–1383. https://doi.org/10.1007/s10021-021-00721-w.
Larson, D., 2007. Growth of three submerged plants below different densities of Nymphoides peltata (S. G. Gmel.) Kuntze. Aquatic Botany 86: 280–284. https://doi.org/10.1016/j.aquabot.2006.10.007.
Lu, J., Z. Wang, W. Xing & G. Liu, 2013. Effects of substrate and shading on the growth of two submerged macrophytes. Hydrobiologia 700: 157–167. https://doi.org/10.1007/s10750-012-1227-5.
Lürling, M., G. van Geest & M. Scheffer, 2006. Importance of nutrient competition and allelopathic effects in suppression of the green alga Scenedesmus obliquus by the macrophytes Chara, Elodea and Myriophyllm. Hydrobiologia 556: 209–220. https://doi.org/10.1007/s10750-005-1168-3.
Morris, K., K. A. Harrison, C. P. E. Bailey & P. I. Boon, 2004. Domain shifts in the aquatic vegetation of shallow urban lakes: the relative roles of low light and anoxia in the catastrophic loss of the submerged angiosperm Vallisneria americana. Marine and Freshwater Research 55: 749–758. https://doi.org/10.1071/MF03193.
Peeters, E. T. H. M., J. P. Van Zuidam, B. G. Van Zuidam, E. H. Van Nes, S. Kosten, P. G. M. Heuts, R. M. M. Roijackers, J. J. C. Netten & M. Scheffer, 2013. Changing weather conditions and floating plants in temperate drainage ditches. Journal of Applied Ecology 50: 585–593. https://doi.org/10.1111/1365-2664.12066.
Phillips, G. L., D. Eminson & B. Moss, 1978. A mechanism to account for macrophyte decline in progressively eutrophicated freshwaters. Aquatic Botany 4: 103–126. https://doi.org/10.1016/j.aquabot.2016.04.004.
Phillips, G., N. Willby & B. Moss, 2016. Submerged macrophyte decline in shallow lakes: what have we learnt in the last forty years? Aquatic Botany 135: 37–45. https://doi.org/10.1016/j.aquabot.2016.04.004.
Pinto, P. T. & I. O’Farrell, 2014. Regime shifts between free-floating plants and phytoplankton: a review. Hydrobiologia 740: 13–24. https://doi.org/10.1007/s10750-014-1943-0.
Roijackers, R. M. M., S. Szabó & M. Scheffer, 2004. Experimental analysis of the competition between algae and duckweed. Archiv für Hydrobiologie 160: 401–412. https://doi.org/10.1127/0003-9136/2004/0160-0401.
Sand-Jensen, K., 1977. Effects of epiphytes on eelgrass photosynthesis. Aquatic Botany 3: 55–63. https://doi.org/10.1016/0304-3770(77)90004-3.
Scheffer, M., S. Szabó, A. Gragnani, E. H. VanNes, S. Rinaldi, N. Kautsky, J. Norberg, R. M. M. Roijackers & R. Franken, 2003. Floating plant dominance as a stable state. Proceedings of the National Academy of Science of the USA 100: 4040–4045. https://doi.org/10.1073/pnas.0737918100.
Silva, W.P. & C. M. D. P. S. Silva, 2018. LAB Fit Curve Fitting Software (Nonlinear Regression and Treatment of Data Program) V 7.2.51 (1999–2020). http://www.labfit.net
Smith, S. D. D., 2014. The role of nitrogene and phosphorus in regulating the dominance of floating and submerged aquatic plants in field mesocosm experiment. Aquatic Botany 112: 1–9. https://doi.org/10.1016/j.aquabot.2013.07.001.
Song, Y., F. Kong, Y. Xue & B. Qin, 2015. Responses of chlorophyll and MDA of Vallisneria natans to nitrogen and phosphorus availability and epiphytic algae. Journal of Freshwater Ecology 30: 85–97. https://doi.org/10.1080/02705060.2014.989554.
Song, Y. Z., J. Q. Wang & Y. X. Gao, 2017. Effects of epiphytic algae on biomass and physiology of Myriophyllum spicatum L. with the increase of nitrogen and phosphorus availability in the water body. Environmental Science and Pollution Research 24: 9548–9555. https://doi.org/10.1007/s11356-017-8604-6.
Szabó, S., E. T. H. M. Peeters, G. Várbíró & G. Borics, 2019. Phenotypic plasticity as a clue for the invasion success of the submerged aquatic plant Elodea nuttallii. Plant Biology 21: 54–63. https://doi.org/10.1111/plb.12918.
Szabó, S., E. T. H. M. Peeters, G. Borics, S. Veres, P. T. Nagy & B. A. Lukács, 2020. The ecophysiological response of two invasive submerged plants to light and nitrogen. Frontiers in Plant Science 10: 1747. https://doi.org/10.3389/fpls.2019.01747.
Szabó, S., G. Koleszár, M. Braun, Z. Nagy, T. T. Vicei & E. T. H. M. Peeters, 2022. Submerged rootless macrophytes sustain stable state against free-floating plants. Ecosystems 25: 17–29. https://doi.org/10.1007/s10021-021-00637-5.
Tóth, V. R., 2013. The effect of periphyton on the light environment and production of Potamogeton perfoliatus L. in the mesotrophic basin of Lake Balaton. Aquatic Sciences 75: 523–534. https://doi.org/10.1007/s00027-013-0297-4.
Van Zuidam, J. P., E. P. Raaphorst & E. T. H. M. Peeters, 2012. The role of propagule banks from drainage ditches dominated by free-floating or submerged plants in vegetation restoration. Restoration Ecology 20: 416–425. https://doi.org/10.1111/j.1526-100X.2011.00784.x.
Vanderstukken, M., S. A. J. Declerck, E. Decaestecker, E. Decaestecker & K. Muylaert, 2014. Long-term allelopathic control of phytoplankton by the submerged macrophyte Elodea nuttallii Planch. Freshwater Biology 59: 930–941. https://doi.org/10.1111/fwb.12316.
Yang, L., H. He, B. Guan, J. Yu, Z. Yao, W. Zhen, C. Yin, Q. Wang, E. Jeppesen & Z. Liu, 2020. Mesocosm experiment reveals a strong positive effect of snail presence on macrophyte growth, resulting from control of epiphyton and nuisance filamentous algae: Implications for shallow lake management. Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2019.135958.
Acknowledgements
This work was supported by the Scientific Board of the University of Nyíregyháza.
Funding
Authors are grateful to the reviewers for their valuable comments.
Author information
Authors and Affiliations
Contributions
SS and EP conceived the idea, SS, AC and GK designed and performed the experiments; SB provided field survey data; SS, SB and EP analysed the data; SS, VO and EP wrote the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Research involving human and animal participants
No human participants and animals were involved in the research.
Additional information
Handling editor: Andre A. Padial
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Szabó, S., Csizmár, A., Koleszár, G. et al. Density-dependent facilitation and inhibition between submerged and free-floating plants. Hydrobiologia 851, 2749–2760 (2024). https://doi.org/10.1007/s10750-024-05491-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-024-05491-9