Abstract
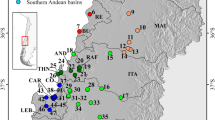
Phylogeographic research is able to identify previously unrecognized units of biodiversity. The aeglid Aegla scamosa is a species that inhabits the Desaguadero basin waterbodies in southern South America. Crabs of the family Aeglidae are especially sensitive to changes in their environment since they have restricted distributions. The aims of our study were to characterize the phylogeography of A. scamosa across its distributional range in Argentina and to identify evolutionarily significant units from these phylogeographic data. We used mitochondrial DNA, nuclear intron, and microsatellite DNA markers to study the genetic diversity, population structure, and phylogeography of the species. Our results indicate that A. scamosa populations are genetically structured in the analyzed sub-basins and that the species comprises at least two independent evolutionarily units: one corresponding to the “North” clade and another coinciding with the “South” clade. We also found a presumptive discrepancy between the information provided by nuclear and mitochondrial markers, that tell different evolutionary stories. Our results highlight the importance of carrying out this kind of study in freshwater fauna, since they may be hiding different evolutionarily significant units as in the case of A. scamosa. The evolutionary complexity of this species should not be ignored in future conservation studies.






Similar content being viewed by others
Data availability
Molecular data used in this work were submitted to GenBank (www.ncbi.nlm.nih.gov). Nuclear intron and mitochondrial gene sequences will be available in the repository, using the access codes MW451770-MW452083. The partial sequences of the genomes used for microsatellite design are found under accession numbers: MN027599; MN027898 (A. platensis) and JF693281.1; JF693283.1; JF693284.1; JF693289.1; JF693280.1; JF693280.1; JF693280.1 (A. araucaniensis).
Code availability
Not applicable.
References
Aguilée, R., D. Claessen & A. Lambert, 2009. Allele fixation in a dynamic metapopulation: founder effects vs refuge effects. Theoretical Population Biology 76: 105–117.
Allendorf, F. W. & G. Luikart, 2009. Conservation and the Genetics of Populations. Wiley, New York.
Avise, J. C., 2000. Phylogeography: The History and Formation of Species. Harvard University Press, Cambridge.
Barber, B. R., J. Xu, M. Pérez-Losada, C. G. Jara, & K. A. Crandall, 2012. Conflicting evolutionary patterns due to mitochondrial introgression and multilocus phylogeography of the Patagonian freshwater crab Aegla neuquensis. PLoS One Public Library of Science 7: e37105.
Bartholomei-Santos, M. L., P. A. Roratto & S. Santos, 2011. High genetic differentiation of Aegla longirostri (Crustacea, Decapoda, Anomura) populations in southern Brazil revealed by multi-loci microsatellite analysis. Genetics and Molecular Research 10: 4133–4146.
Barraclough, T. G., M. Hughes, N. Ashford-Hodges, N. & Fujisawa, 2009. Inferring evolutionarily significant units of bacterial diversity from broad environmental surveys of single-locus data. Biology Letters 5(3): 425–428. https://doi.org/10.1098/rsbl.2009.0091
Beaumont, M. A. & B. Rannala, 2004. The Bayesian revolution in genetics. Nature Reviews Genetics 5: 251–261.
Bivand, R., M. Altman, L. Anselin, R. Assunção, O. Berke, G. Blanchet, M. Carvalho, B. Christensen, Y. Chun, & C. Dormann, 2022. Package ‘spdep’: spatial dependence: weighting schemes, statistics.
Bond-Buckup, G. & L. Buckup, 1994. A família Aeglidae (Crustacea, Decapoda, Anomura). Arquivos de Zoologia 32: 159–346.
Bromilow, S. M., & F. A. H. Sperling, 2011. Phylogeographic signal variation in mitochondrial DNA among geographically isolated grassland butterflies. Journal of Biogeography Wiley Online Library 38: 299–310.
Brown, W. M., 1983. Evolution of animal mitochondrial DNA. Evolution of genes and proteins Sinauer 62–88.
Burland, T. G., 2000. DNASTAR’s Lasergene Sequence Analysis Software Bioinformatics Methods and Protocols. Springer, New York: 71–91.
Cazenave, H. W., 2015. LA CUENCA DEL RÍO DESAGUADERO: un caso de desertificación por acción antropica. InterEspaço: Revista de Geografia e Interdisciplinaridade 1: 225–236.
Collen, B., F. Whitton, E. E. Dyer, J. E. M. Baillie, N. Cumberlidge, W. R. T. Darwall, C. Pollock, N. I. Richman, A. Soulsby & M. Böhm, 2014. Global patterns of freshwater species diversity, threat and endemism. Global Ecology and Biogeography 23: 40–51.
Colombo, F., P. Busquets, E. Ramos, J. Vergés & D. Ragona, 2000. Quaternary alluvial terraces in an active tectonic region: the San Juan River valley, Andean ranges, San Juan Province, Argentina. Journal of South American Earth Sciences 13: 611–626.
Crivellaro, M. S., B. L. Zimmermann, M. L. Bartholomei-Santos, K. A. Crandall, M. Pérez-Losada, G. Bond-Buckup & S. Santos, 2018. Looks can be deceiving: species delimitation reveals hidden diversity in the freshwater crab Aegla longirostri (Decapoda: Anomura). Zoological Journal of the Linnean Society 182: 24–37.
Darwall, W., K. Smith, D. Allen, M. Seddon, G. M. Reid, V. Clausnitzer, & V. J. Kalkman, 2009. Freshwater biodiversity: a hidden resource under threat. Wildlife in a changing world–An Analysis of the 2008 IUCN Red List of Threatened Species by: IUCN, Gland, Switzerland 43.
Darriba, D., G. L. Taboada, R. Doallo, & D. Posada, 2012. jModelTest 2: more models, new heuristics and parallel computing. Nature methods Nature Publishing Group 9: 772.
de Siqueira Bueno, S. L., R. M. Shimizu, & J. C. B. Moraes, 2016. A remarkable anomuran: the taxon Aegla Leach, 1820. Taxonomic remarks, distribution, biology, diversity and conservation A global overview of the conservation of freshwater decapod crustaceans. Springer: 23–64.
Dellicour, S., & J.-F. Flot, 2015. Delimiting species-poor data sets using single molecular markers: a study of barcode gaps, haplowebs and GMYC. Systematic Biology Oxford University Press 64: 900–908.
Dray, S., 2020. Moran’s Eigenvector Maps and related methods for the spatial multiscale analysis of ecological data. dim (mafragh $ flo) 1: 56.
Dray, S., D. Bauman, G. Blanchet, D. Borcard, S. Clappe, G. Guenard, T. Jombart, G. Larocque, P. Legendre, & N. Madi, 2021. Adespatial: Multivariate Multiscale Spatial Analysis (v. 0.3-14).
Drummond, A. J., M. A. Suchard, D. Xie, & A. Rambaut, 2012. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Molecular biology and evolution Oxford University Press 29: 1969–1973.
Dudgeon, D., A. H. Arthington, M. O. Gessner, Z.-I. Kawabata, D. J. Knowler, C. Lévêque, R. J. Naiman, A.-H. Prieur-Richard, D. Soto & M. L. J. Stiassny, 2006. Freshwater biodiversity: importance, threats, status and conservation challenges. Biological Reviews 81: 163–182.
Earl, D. A., 2012. STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conservation Genetics Resources 4: 359–361.
Excoffier, L., & H. Lischer, 2006. An integrated software package for population genetics data analysis. Computational and Molecular Population Genetics Lab (CMPG), Institute of Zoology, University of Berne, Switzerland.
Frankham, R., S. E. J. D. Ballou, D. A. Briscoe & J. D. Ballou, 2002. Introduction to Conservation Genetics. Cambridge University Press, Cambridge.
Frankham, R., J. D. Ballou, K. Ralls, M. Eldridge, M. R. Dudash, C. B. Fenster, R. C. Lacy & P. Sunnucks, 2017. Genetic Management of Fragmented Animal and Plant Populations. Oxford University Press, Oxford.
Freeland, J., 2001. Molecular Ecology. Wiley, New York.
Fujisawa, T., & T. G. Barraclough, 2013. Delimiting species using single-locus data and the Generalized Mixed Yule Coalescent approach: a revised method and evaluation on simulated data sets. Systematic biology Oxford University Press 62: 707–724.
Guo, B., & L. Kong, 2022. Comparing the Efficiency of Single-Locus Species Delimitation Methods within Trochoidea (Gastropoda: Vetigastropoda). Genes MDPI 13: 2273.
Hudson, R. R. & M. Turelli, 2003. Stochasticity overrules the “three-times rule”: genetic drift, genetic draft, and coalescence times for nuclear loci versus mitochondrial DNA. Evolution 57: 182–190.
Hughes, J. M. & M. J. Hillyer, 2003. Patterns of connectivity among populations of Cherax destructor (Decapoda: Parastacidae) in western Queensland, Australia. Marine and Freshwater Research 54: 587–596.
Hutchison, D. W. & A. R. Templeton, 1999. Correlation of pairwise genetic and geographic distance measures: inferring the relative influences of gene flow and drift on the distribution of genetic variability. Evolution 53: 1898–1914.
Klunzinger, M. W., M. Lopes-Lima, A. Gomes-dos-Santos, E. Froufe, A. J. Lymbery, & L. Kirkendale, 2020. Phylogeographic study of the West Australian freshwater mussel, Westralunio carteri, uncovers evolutionarily significant units that raise new conservation concerns. Hydrobiologia 1–14.
Kopelman, N. M., J. Mayzel, M. Jakobsson, N. A. Rosenberg & I. Mayrose, 2015. Clumpak: a program for identifying clustering modes and packaging population structure inferences across K. Molecular Ecology Resources 15: 1179–1191.
Kumar, S., G. Stecher & K. Tamura, 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution Society for Molecular Biology and Evolution 33: 1870–1874.
Larkin, M. A., G. Blackshields, N. P. Brown, R. Chenna, P. A. McGettigan, H. McWilliam, F. Valentin, I. M. Wallace, A. Wilm & R. Lopez, 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947–2948.
López, H. L., R. C. Menni, M. Donato & A. M. Miquelarena, 2008. Biogeographical revision of Argentina (Andean and Neotropical Regions): an analysis using freshwater fishes. Journal of Biogeography 35: 1564–1579.
Loretán, G., E. C. Rueda, J. M. Cabrera, M. Pérez-Losada, P. A. Collins & F. Giri, 2020. Geographical isolation and restricted gene flow drive speciation of Aegla singularis (Decapoda: Anomura: Aeglidae) in southern South America. Biological Journal of the Linnean Society 129: 177–189.
Matschiner, M. & W. Salzburger, 2009. TANDEM: integrating automated allele binning into genetics and genomics workflows. Bioinformatics 25: 1982–1983.
McCauley, E., W. G. Wilson, & A. M. de Roos, 1993. Dynamics of age-structured and spatially structured predator-prey interactions: individual-based models and population-level formulations. The American Naturalist University of Chicago Press 142: 412–442.
Metz, S., J. M. Cabrera, E. Rueda, F. Giri, & P. Amavet, 2016. FullSSR: microsatellite finder and primer designer. Advances in Bioinformatics.
Moraes, J. C. B., M. Terossi, R. C. Buranelli, M. Tavares, F. L. Mantelatto, & S. L. D. S. Bueno, 2016. Morphological and molecular data reveal the cryptic diversity among populations of Aegla paulensis (Decapoda, Anomura, Aeglidae), with descriptions of four new species and comments on dispersal routes and conservation status. Zootaxa 4193: 1–48.
Moritz, C., 1994. Defining ‘evolutionarily significant units’ for conservation. Trends in Ecology & Evolution 9: 373–375.
Moritz, C. & D. P. Faith, 1998. Comparative phylogeography and the identification of genetically divergent areas for conservation. Molecular Ecology 7: 419–429.
Papadopoulou, A., I. Anastasiou, F. Spagopoulou, M. Stalimerou, S. Terzopoulou, A. Legakis, & A. P. Vogler, 2011. Testing the species–genetic diversity correlation in the Aegean archipelago: toward a haplotype-based macroecology? The American Naturalist University of Chicago Press Chicago, IL 178: 241–255.
Peakall, R. O. D. & P. E. Smouse, 2006. GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Molecular Ecology 6: 288–295.
Pérez-Losada, M., C. G. Jara, G. Bond-Buckup, M. L. Porter & K. A. Crandall, 2002. Phylogenetic position of the freshwater anomuran family Aeglidae. Journal of Crustacean Biology 22: 670–676.
Pérez-Losada, M., G. Bond-Buckup, C. G. Jara & K. A. Crandall, 2004. Molecular systematics and biogeography of the southern South American freshwater “crabs” Aegla (Decapoda: Anomura: Aeglidae) using multiple heuristic tree search approaches. Systematic Biology Society of Systematic Zoology 53: 767–780.
Pérez-Losada, M., G. Bond-Buckup, C. G. Jara & K. A. Crandall, 2009. Conservation assessment of southern South American freshwater ecoregions on the basis of the distribution and genetic diversity of crabs from the genus Aegla. Conservation Biology 23: 692–702.
Pérez-Losada, M., J. Xu, C. G. Jara, & K. A. Crandall, 2011. Comparing phylogeographic patterns across the Patagonian Andes in two freshwater crabs of the genus Aegla (Decapoda: Aeglidae). Genetic Analysis 291–303.
Pérez-Moreno, J. L., G. Balázs, B. Wilkins, G. Herczeg & H. D. Bracken-Grissom, 2017. The role of isolation on contrasting phylogeographic patterns in two cave crustaceans. BMC Evolutionary Biology 17: 1–13.
Pons, J., T. G. Barraclough, J. Gomez-Zurita, A. Cardoso, D. P. Duran, S. Hazell, S. Kamoun, W. D. Sumlin & A. P. Vogler, 2006. Sequence-based species delimitation for the DNA taxonomy of undescribed insects. Systematic Biology 55: 595–609.
Pritchard, J. K., W. Wen, & D. Falush, 2003. Documentation for STRUCTURE software: Version 2. Citeseer.
R Development Core Team (2011). R Language. R Foundation for Statistical Computing, Vienna, Austria. http://CRAN.R-Project.org/doc/manuals/R-lang.html
Rambaut, A., 2009. FigTree, version 1.3. 1. Computer program distributed by the author, website: http://tree.bio.ed.ac.uk/software/figtree/. Accessed January 4, 2011.
Rambaut, A., A. J. Drummond, D. Xie, G. Baele, & M. A. Suchard, 2018. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic biology Oxford University Press 67: 901.
Revenga, C., I. Campbell, R. Abell, P. De Villiers & M. Bryer, 2005. Prospects for monitoring freshwater ecosystems towards the 2010 targets. Philosophical Transactions of the Royal Society B: Biological Sciences the Royal Society London 360: 397–413.
Richman, N. I., M. Böhm, S. B. Adams, F. Alvarez, E. A. Bergey, J. J. S. Bunn, Q. Burnham, J. Cordeiro, J. Coughran & K. A. Crandall, 2015. Multiple drivers of decline in the global status of freshwater crayfish (Decapoda: Astacidea). Philosophical Transactions of the Royal Society B: Biological Sciences the Royal Society 370: 20140060.
Robalo, J. I., I. Doadrio, A. Valente & V. C. Almada, 2007. Identification of ESUs in the critically endangered Portuguese minnow Chondrostoma lusitanicum Collares-Pereira 1980, based on a phylogeographical analysis. Conservation Genetics 8: 1225–1229.
Rowe, G., M. Sweet & T. J. C. Beebee, 2017. An Introduction to Molecular Ecology. Oxford University Press, Oxford.
Rozas, J., A. Ferrer-Mata, J. C. Sánchez-DelBarrio, S. Guirao-Rico, P. Librado, S. E. Ramos-Onsins & A. Sánchez-Gracia, 2017. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Molecular Biology and Evolution 34: 3299–3302. https://doi.org/10.1093/molbev/msx248/4161815.
Ruiz-García, M., M. F. Jaramillo & J. M. Shostell, 2019. Mitochondrial phylogeography of kinkajous (Procyonidae, Carnivora): maybe not a single ESU. Journal of Mammalogy 100: 1631–1652.
Santos, S., G. Bond-Buckup, M. Perez-Losada, M. L. Bartholomei-Santos & L. Buckup, 2009. Aegla manuinflata, a new species of freshwater anomuran (Decapoda: Anomura: Aeglidae) from Brazil, determined by morphological and molecular characters. Zootaxa 2088: 31–40.
Sherman, G. E., T. Sutton, R. Blazek, S. Holl, O. Dassau, B. Morely, T. Mitchell, & L. Luthman, 2014. QGIS Geographic Information System. Open Source Geospatial Foundation Project. Disponível em:. Acesso em 25:
Stefani, F., S. Zaccara, G. B. Delmastro & M. Buscarino, 2011. The endangered white-clawed crayfish Austropotamobius pallipes (Decapoda, Astacidae) east and west of the Maritime Alps: a result of human translocation? Conservation Genetics 12: 51–60.
Stevens, V. M., É. Leboulengé, R. A. Wesselingh & M. Baguette, 2006. Quantifying functional connectivity: experimental assessment of boundary permeability for the natterjack toad (Bufo calamita). Oecologia 150: 161–171.
Suvires, G. M., R. Mon & A. A. Gutiérrez, 2012. Tectonic effects on the drainage disposition in mountain slopes and orogen forelands. A case study: the Central Andes of Argentina. Brazilian Journal of Geology 42: 229–239.
Taylor, C. A., G. A. Schuster, J. E. Cooper, R. J. DiStefano, A. G. Eversole, P. Hamr, H. H. Hobbs III., H. W. Robison, C. E. Skelton & R. F. Thoma, 2007. A reassessment of the conservation status of crayfishes of the United States and Canada after 10+ years of increased awareness. Fisheries 32: 372–389.
Team, R. C., 2021. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Toews, D. P. L. & A. Brelsford, 2012. The biogeography of mitochondrial and nuclear discordance in animals. Molecular Ecology 21: 3907–3930.
Tsai, Y. E., 2011. PhyloGeoViz: a web-based program that visualizes genetic data on maps. Molecular Ecology Resources 11: 557–561.
Tumini, G., F. Giri, V. Williner, P. A. Collins & J. J. Morrone, 2019. Selecting and ranking areas for conservation of Aegla (Crustacea: Decapoda: Anomura) in southern South America integrating biogeography, phylogeny and assessments of extinction risk. Aquatic Conservation: Marine and Freshwater Ecosystems 29: 693–705.
van Oosterhout, C., W. F. Hutchinson, D. P. M. Wills & P. F. Shipley, 2003. Micro-checker. Department of Computer Sciences, University of Hull, Hull.
Waples, R. S., 1991. Pacific salmon, Oncorhynchus spp., and the definition of" species" under the Endangered Species Act. Marine Fisheries Review 53: 11–22.
Wright, S., 1943. Isolation by distance. Genetics Genetics Society of America 28: 114.
Xu, J., M. Pérez-losada, C. G. Jara & K. A. Crandall, 2009. Pleistocene glaciation leaves deep signature on the freshwater crab Aegla alacalufi in Chilean Patagonia. Molecular Ecology 18: 904–918.
ZAKšEK, V., B. Sket, S. Gottstein, D. Franjević & P. Trontelj, 2009. The limits of cryptic diversity in groundwater: phylogeography of the cave shrimp Troglocaris anophthalmus (Crustacea: Decapoda: Atyidae). Molecular Ecology 18: 931–946.
Zhou, R., X. Wang, J. Wan & N. Xiong, 2020. EDM-fuzzy: an Euclidean distance based multiscale fuzzy entropy technology for diagnosing faults of industrial systems. IEEE Transactions on Industrial Informatics IEEE 17: 4046–4054.
Zink, R. M. & G. F. Barrowclough, 2008. Mitochondrial DNA under siege in avian phylogeography. Molecular Ecology 17: 2107–2121.
Zimmermann, B. L., I. Buzatto, S. Santos, F. Giri, F. Teixeira de Mello, K. A. Crandall, M. Pérez‐Losada, & M. L. Bartholomei‐Santos, 2021. Entangled Aeglidae (Decapoda, Anomura): Additional evidence for cryptic species. Zoologica Scripta Wiley Online Library 50: 473–484.
Acknowledgements
Gisela Loretán is a fellow of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) from Argentina. We are grateful to FonCyT-ANCyP (Fondo para la Investigación Científica y Tecnológica—Agencia Nacional de Promoción Científica y Tecnológica), PICT 2014-3502, for funding this research. We also thank the Computational Biology Institute (The George Washington University) and his Director Dr. Crandall for allowing us to carry out part of this work and for his predisposition and human support.
Funding
FonCyT-ANCyP (Fondo para la Investigación Científica y Tecnológica—Agencia Nacional de Promoción Científica y Tecnológica), PICT Proyect 2014-3502. Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), in charge of financing salaries.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
Not applicable.
Consent to participate
All authors made a significant contribution for this study, and they have actively participated in the manuscript writing.
Consent for publication
All authors approve the submission of this manuscript and its publication in Hydrobiologia.
Research involving humans and/or animals
Not applicable.
Additional information
Handling editor: Diego Fontaneto
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

10750_2023_5264_MOESM3_ESM.tiff
Supplementary file3 Fig. 9 Graphic of the mantel test among EFα1 intron FST values and geographic distances. (TIFF 5311 KB)
10750_2023_5264_MOESM5_ESM.tiff
Supplementary file5 Fig. 11 Graphic of the mantel test among microsatellites FST values and geographic distances. (TIFF 5311 KB)
10750_2023_5264_MOESM6_ESM.tiff
Supplementary file6 Fig. 12 Micro mantel correlations (Iglesia-Rodeo-Aguas Negras-Cuesta del Viento populations) among COII FST values and geographic distances. (TIFF 5311 KB)
10750_2023_5264_MOESM7_ESM.tiff
Supplementary file7 Fig. 13 Micro mantel correlations (Iglesia-Rodeo-Aguas Negras-Cuesta del Viento populations) among EFα1 intron FST values and geographic distances. (TIFF 5311 KB)
10750_2023_5264_MOESM8_ESM.tiff
Supplementary file8 Fig. 14 Micro mantel correlations (Iglesia-Rodeo-Aguas Negras-Cuesta del Viento populations) among COII and nuclear distances FST’s (TIFF 5311 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Loretán, G., Giri, F., Cabrera, J.M. et al. Phylogeography reveals complex historical processes and different evolutionarily significant units in Aegla scamosa freshwater crabs. Hydrobiologia 850, 3627–3644 (2023). https://doi.org/10.1007/s10750-023-05264-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05264-w