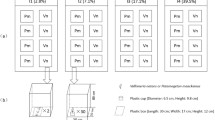
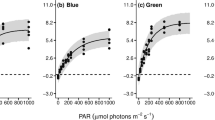
Abstract
The biomass and composition of autotrophic communities in the littoral zone are mainly affected by light availability. In a field study, the spectral attenuation of periphyton was assessed. Periphyton absorbed more light in the red than in the infrared spectral range, resulting in a lower red to infrared ratio (~0.3 during the most active period of periphyton accumulation, compared with 0.9–1 otherwise). The lowest red to infrared ratio was detected in the upper 20–40 cm of the water column. Epiphytic algae are therefore found to affect not only the quantity but also the quality of light passing through periphyton. Acclimation of Potamogeton perfoliatus L. plantlets to such infrared-enriched light was also studied in the laboratory. During leaf morphogenesis, lower red to infrared ratio light was associated with increased leaf area via the growth of existing (+85%) and the production of new leaves. Intensified internode length growth (+130%) was also observed. Post morphogenesis, no leaf or internode growth was observed and new shoot production was also intensive. Leaf photochemical activity did not significantly differ between groups or treatments. Results suggest that periphyton could trigger shade-tolerance (leaf growth), shade-avoidance (internode growth), and morphogenetic (branch production from axillary buds) adaptations in macrophytes.






Similar content being viewed by others
References
Ács, É., N. M. Reskóné, K. Szabó, G. Taba & K. T. Kiss, 2005. Application of epiphytic diatoms in water quality monitoring of Lake Velence-recommendations and assignments. Acta Botanica Hungarica 47: 211–223.
Arenas, F., R. M. Viejo & C. Fernández, 2002. Density-dependent regulation in an invasive seaweed: responses at plant and modular levels. Journal of Ecology 90: 820–829.
Asaeda, T., M. Sultana, J. Manatunge & T. Fujino, 2004. The effect of epiphytic algae on the growth and production of Potamogeton perfoliatus L. in two light conditions. Environmental and Experimental Botany 52: 225–238.
Ballaré, C. L., 1999. Keeping up with the neighbours: phytochrome sensing and other signalling mechanisms. Trends in Plant Science 4: 97–102.
Barthélémy, D. & Y. Caraglio, 2007. Plant architecture: a dynamic, multilevel and comprehensive approach to plant form, structure and ontogeny. Annals of Botany 99: 375.
Bécares, E., J. Gomá, M. Fernández-Aláez, C. Fernández-Aláez, S. Romo, M. R. Miracle, A. Ståhl-Delbanco, L. A. Hansson, M. Gyllström & W. J. Van de Bund, 2008. Effects of nutrients and fish on periphyton and plant biomass across a European latitudinal gradient. Aquatic Ecology 42: 561–574.
Cattaneo, A., G. Galanti & S. Gentinetta, 1998. Epiphytic algae and macroinvertebrates on submerged and floating leaved macrophytes in an Italian lake. Freshwater Biology 39: 725–740.
Crawley, M. J., 2009. Life History and Environment Plant Ecology. Blackwell Publishing Ltd, Oxford: 73–131.
de Kroon, H., H. Huber, J. F. Stuefer & J. M. van Groenendael, 2005. A modular concept of phenotypic plasticity in plants. New Phytologist 166: 73–82.
Eilers, P. H. C. & J. C. H. Peeters, 1988. A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecological Modelling 42: 199–215.
Elliott, R. J. & A. G. Porter, 1971. A rapid cadmium reduction method for the determination of nitrate in bacon and curing brines. Analyst 96: 522–527.
Franklin, K. A., 2008. Shade avoidance. New Phytologist 179: 930–944.
Franklin, K. A. & G. C. Whitelam, 2005. Phytochromes and shade-avoidance responses in plants. Annals of Botany 96: 169–175.
Gales, M. E., E. C. Julian & R. C. Kroner, 1966. Method for quantitative determination of total phosphorus in water. Journal of American Water Works Association 58: 1363–1368.
Gross, E. M., 2003. Allelopathy of aquatic autotrophs. Critical Reviews in Plant Sciences 22: 313–339.
Hutchings, M. J., & M. Mogie, 1990. The Spatial Structure of Clonal Plants: Control and Consequences. Clonal Growth in Plants: Regulation and Function. SPB Academic Publishing, The Hague 57: 76.
Jones, J. I., B. Moss, J. W. Eaton & J. O. Young, 2000. Do submerged aquatic plants influence periphyton community composition for the benefit of invertebrate mutualists? Freshwater Biology 43: 591–604.
Kami, C., S. Lorrain, P. Hornitschek & C. Fankhauser, 2010. Chapter two-light-regulated plant growth and development. Current Topics in Developmental Biology 91: 29–66.
Kendrick, R. E. & G. H. M. Kronenberg, 1994. Photomorphogenesis in Plants. Springer, New York.
Kirk, J. T. O., 1994. Light and Photosynthesis in Aquatic Ecosystems. Cambridge University Press, Cambridge.
Lacoul, P. & B. Freedman, 2006. Environmental influences on aquatic plants in freshwater ecosystems. Environmental Reviews 14: 89–136.
Liboriussen, L. & E. Jeppesen, 2003. Temporal dynamics in epipelic, pelagic and epiphytic algal production in a clear and a turbid shallow lake. Freshwater Biology 48: 418–431.
Liboriussen, L. & E. Jeppesen, 2009. Periphyton biomass, potential production and respiration in a shallow lake during winter and spring. Hydrobiologia 632: 201–210.
Mackereth, F. J., J. Heron & J. F. Talling, 1978. Water Analysis: Some Revised Methods for Limnologists. Freshwater Biological Association, Ambleside.
Mathieu, A., P. H. Courn de, V. Letort, D. Barthélémy & P. De Reffye, 2009. A dynamic model of plant growth with interactions between development and functional mechanisms to study plant structural plasticity related to trophic competition. Annals of Botany 103: 1173.
Monro, K. & A. G. B. Poore, 2005. Light quantity and quality induce shade-avoiding plasticity in a marine macroalga. Journal of evolutionary biology 18: 426–435.
Mooney, H. A. & J. R. Ehleringer, 2009. Photosynthesis Plant Ecology. Blackwell Publishing Ltd, Wageningen: 1–27.
Neff, M. M., C. Fankhauser & J. Chory, 2000. Light: an indicator of time and place. Genes & Development 14: 257–271.
Newell, B. S., B. Morgan & J. Cundy, 1967. The determination of urea in seawater. Journal of Marine Research 25: 201–202.
Nõges, T., H. Luup & T. Feldmann, 2010. Primary production of aquatic macrophytes and their epiphytes in two shallow lakes (Peipsi and Võrtsjärv) in Estonia. Aquatic Ecology 44: 83–92.
Quail, P. H., 2002. Phytochrome photosensory signalling networks. Nature Reviews Molecular Cell Biology 3: 85–93.
R Development Core Team, 2012. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/.
Ritchie, R. J., 2008. Universal chlorophyll equations for estimating chlorophylls a, b, c, and d and total chlorophylls in natural assemblages of photosynthetic organisms using acetone, methanol, or ethanol solvents. Photosynthetica 46: 115–126.
Robin, C. H., M. J. M. Hay, P. C. D. Newton & D. H. Greer, 1994. Effect of light quality (red: far-red ratio) at the apical bud of the main stolon on morphogenesis of Trifolium repens L. Annals of Botany 74: 119–123.
Scheffer, M. & E. Nes, 2007. Shallow lakes theory revisited: various alternative regimes driven by climate, nutrients, depth and lake size. Hydrobiologia 584: 455–466.
Scheffer, M., S. Hosper, M. Meijer, B. Moss & E. Jeppesen, 1993. Alternative equilibria in shallow lakes. Trends in Ecology & Evolution 8: 275–279.
Shinomura, T., A. Nagatani, H. Hanzawa, M. Kubota, M. Watanabe & M. Furuya, 1996. Action spectra for phytochrome A-and B-specific photoinduction of seed germination in Arabidopsis thaliana. Proceedings of the National Academy of Sciences 93: 8129–8133.
Smith, H., 2000. Phytochromes and light signal perception by plants—an emerging synthesis. Nature 407: 585–591.
Smith, H. & G. C. Whitelam, 1997. The shade avoidance syndrome: multiple responses mediated by multiple phytochromes. Plant, Cell & Environment 20: 840–844.
Strayer, D. L. & S. E. Findlay, 2010. Ecology of freshwater shore zones. Aquatic Sciences-Research Across Boundaries 72: 127–163.
Sultana, M., T. Asaeda, M. Ekram Azim & T. Fujino, 2010. Morphological responses of a submerged macrophyte to epiphyton. Aquatic Ecology 44: 73–81.
Talarico, L. & G. Maranzana, 2000. Light and adaptive responses in red macroalgae: an overview. Journal of Photochemistry and Photobiology B: Biology 56: 1–11.
Tilman, D., 2009. Mechanisms of Plant Competition Plant Ecology. Blackwell Publishing Ltd, Oxford: 239–261.
Tóth, V. R., 2013. The effect of periphyton on the light environment and production of Potamogeton perfoliatus L. in the mesotrophic basin of Lake Balaton. Aquatic Sciences 75: 523–534.
Tóth, V. R. & Á. Vári, 2013. Impact of habitat environment on Potamogeton perfoliatus L. morphology and its within-plant variability in Lake Balaton. Annales de Limnologie - International Journal of Limnology 49: 149–155.
Tóth, V. R., Á. Vári & S. Luggosi, 2011. Morphological and photosynthetic acclimation of Potamogeton perfoliatus to different environments in Lake Balaton. Oceanological and Hydrobiological Studies 40: 43–51.
Valladares, F. & Ü. Niinemets, 2008. Shade tolerance, a key plant feature of complex nature and consequences. Annual Review of Ecology, Evolution, and Systematics 39: 237–257.
Vári, Á., V. R. Tóth & P. Csontos, 2010. Comparing the morphology of Potamogeton perfoliatus L. along environmental gradients in Lake Balaton (Hungary). Annales de Limnologie-International Journal of Limnology 46: 111–119.
Vis, C., C. Hudon & R. Carignan, 2006. Influence of the vertical structure of macrophyte stands on epiphyte community metabolism. Canadian Journal of Fisheries and Aquatic Sciences 63: 1014–1026.
Vis, C., C. Hudon, R. Carignan & P. Gagnon, 2007. Spatial analysis of production by macrophytes, phytoplankton and epiphyton in a large river system under different water-level conditions. Ecosystems 10: 293–310.
Weisner, S. E. B., J. A. Strand & H. Sandsten, 1997. Mechanisms regulating abundance of submerged vegetation in shallow eutrophic lakes. Oecologia 109: 592–599.
Westoby, M., D. S. Falster, A. T. Moles, P. A. Vesk & I. J. Wright, 2002. Plant ecological strategies: some leading dimensions of variation between species. Annual Review of Ecology and Systematic 33: 125–159.
Wetzel, R. G., 1975. Limnology. Saunders, Philadelphia.
Whitelam, G. C. & K. J. Halliday, 1999. Photomorphogenesis: phytochrome takes a partner! Current biology 9: R225–R227.
Zimba, P. V. & M. S. Hopson, 1997. Quantification of epiphyte removal efficiency from submersed aquatic plants. Aquatic Botany 58: 173–179.
Acknowledgments
This project was supported by TÁMOP-4.2.2.A-11/1/KONV-2012-0038. SCJP is grateful for the financial support of GIONET, funded by the European Commission, Marie Curie Programme Initial Training Network, Grant Agreement PITN-GA-2010-264509.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Sidinei M. Thomaz
Rights and permissions
About this article
Cite this article
Tóth, V.R., Palmer, S.C.J. Acclimation of Potamogeton perfoliatus L. to periphyton accumulation-induced spectral changes in irradiance. Hydrobiologia 766, 293–304 (2016). https://doi.org/10.1007/s10750-015-2462-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2462-3