Abstract
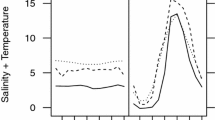
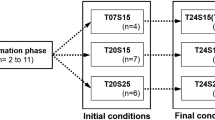
Organisms living in temporary and shallow wetlands are adapted to survive in very fluctuating and unpredictable conditions and might help us to understand life cycle strategies and plasticity in the context of global warming. Despite the importance of Arctodiaptomus salinus in these systems, little is known about the effect of temperature on its population dynamics. Through an individual-based experimental protocol, we studied the effect of this factor and food on its reproduction. This approach has revealed a large range of variability in reproductive parameters in all the experimental conditions. Temperature positively affected egg production and negatively longevity, but did not affect clutch size. Under unsuitable food conditions, the clutch size decreased and the inter-clutch period increased, and when the food conditions improved, the number of eggs increased gradually in every clutch. Eggs from the same clutch hatched synchronously. In contrast, there were significant differences between the hatching times of clutches from different females and between those of the same female. The observed individual variability increased when temperature moved away from the medium values. The thermal tolerance threshold for A. salinus development might be around 25–29°C. Since water pond is close to this thermal limit for long periods of time, an increment of temperature because of global warming might have dramatic consequences on this population. The individual-based experimental approach of this study provides useful information to construct realistic individual-based models, which will help us to better understand the population-level consequences of individual variability in A. salinus reproduction.








Similar content being viewed by others
References
Alcorlo, P., 1999. Redes tróficas en lagunas salinas temporales de la comarca de Los Monegros (Zaragoza). PhD thesis, Universidad Autónoma de Madrid
Alcorlo, P., A. Baltanás & C. Montes, 2001. Food-web structure in two shallow salt lakes in Los Monegros (NE Spain): energetic vs dynamic constraints. Hydrobiologia 466: 307–316.
Alonso, M., 1998. Las lagunas de la España peninsular. Limnetica 15: 1–176.
Armengol, J., M. Estrada, A. Guiset, et al., 1975. Observaciones limnológicas en las lagunas de la Mancha. Boletín de la Estación Central de Ecología 4: 11–27.
Austin, M. P., 1987. Models for the analysis of species response to environmental gradients. Vegetatio 69: 35–45.
Berger, I. & G. Maier, 2001. The mating and reproductive biology of the freshwater planktonic calanoid copepod Eudiaptomus gracilis. Freshwater Biology 46: 787–794.
Birley, M. H., 1979. The estimation and simulation of variable developmental period, with application to the mosquito Aedes aegypti (L.). Research on Population Ecology 21: 68–80.
Boronat Chirivella, M. D., 2003. Distribución de los microcrustáceos en lagunas de Castilla la Mancha. Ciclos estacionales y migración vertical en lagunas cársticas estratificadas. PhD thesis, Universitat de Valencia.
Caramujo, M. J. & M. J. Boavida, 1999. Characteristics of the reproductive cycles and development times of Copidodiaptomus numidicus (Copepoda: Calanoida) and Acanthocyclops robustus (Copepoda: Cyclopoida). Journal of Plankton Research 21: 1765–1778.
Carlotti, F. & S. Nival, 1991. Individual variability of development in laboratory-reared Temora stylifera copepodites: consequences for the population dynamics and interpretation in the scope of growth and development rules. Journal of Plankton Research 13: 801–813.
Castro, M. C., 2004. Caracterización limnológica y variabilidad temporal de la comunidad planctónica en Laguna Honda (Jaén). PhD thesis, Universidad de Jaén.
Castro, M. C., M. Rivera, M. Crespo, J. M. Martín-García & F. Guerrero, 2003. Morphological and sedimentological characterization of Honda temporary lake (southern Spain). Limnetica 22: 147–154.
Chinnery, F. E. & J. A. Williams, 2004. The influence of temperature and salinity on Acartia (Copepoda: Calanoida) nauplii survival. Marine Biology 145: 733–738.
Cooley, J. M. & C. K. Minns, 1978. Prediction of egg development times of freshwater copepods. Journal of the Fisheries Research Board of Canada 35: 1322–1329.
Devreker, D., S. Souissi & L. Seuront, 2005. Effects of chlorophyll concentration and temperature variation on the reproduction and survival of Temora longicornis (Copepoda, Calanoida) in the Eastern English Channel. Journal of Experimental Marine Biology and Ecology 318: 145–162.
Devreker, D., S. Souissi, G. Winkler, J. Forget-Leray & F. Leboulenger, 2009. Effects of salinity, temperature and individual variability on the reproduction of Eurytemora affinis (Copepoda: Calanoida) from the Seine estuary: a laboratory study. Journal of Experimental Marine Biology and Ecology 368: 113–123.
Donaghay, P. L., 1985. An experimental test of the relative significance of food quality and past feeding history to limitation of egg production of the estuarine copepod Acartia tonsa. Archiv für Hydrobiologie 21: 235–245.
Dussart, B. H. & D. Defaye, 1995. Introduction to the Copepoda. In Dumont, H. J. F. (ed.), Guides to the Identification of the Microinvertebrates of the Continental Waters of the World, Vol. 7. SPB, Amsterdam.
Elster, H. J., M. Hawary, R. Schroeder & I. Schwerbel, 1960. Population dynamics of zooplankton in the Nozha-Hydrodome near Alexandria. Alexandria Institute of Hydrobiology Notes and Memoires 50: 1–27.
Frisch, D. & B. Santer, 2004. Temperature-induced responses of a permanent-pond and a temporary-pond cyclopoid copepod: a link to habitat predictability? Evolutionary Ecology Research 6: 541–553.
Grimm, V. & S. F. Railsback, 2005. Individual-Based Modelling and Ecology. Princeton University Press, Princeton.
Guerrero, F. & M. C. Castro, 1997. Chlorophyll a of size-fractioned phytoplankton at a temporary hypersaline lake. International Journal of Salt Lake Research 5: 253–260.
Guerrero, F., J. M. Blanco & V. Rodríguez, 1994. Temperature-dependent development in marine copepods: a comparative analysis of models. Journal of Plankton Research 16: 95–103.
Guerrero, F., S. Nival & P. Nival, 1997. Egg production and viability in Centropages typicus: a laboratory study on the effect of food concentration. Journal of the Marine Biological Association of the United Kingdom 77: 257–260.
Guisan, A., T. C. Edwards Jr. & T. Hastie, 2002. Generalized linear and generalized additive models in studies of species distributions: setting the scene. Ecological Modelling 157: 89–100.
Gutelmacher, B. L., 1986. Metabolism of plankton as a single whole: trophometabolic interaction between zoo- and phytoplankton. Nauka, Leningrad.
Hansen, A. & B. Santer, 1995. The influence of food resources on the development, survival and reproduction of the two cyclopoid copepods: Cyclops vicinus and Mesocyclops leuckarti. Journal of Plankton Research 17: 631–646.
Hastie, T. J. & R. J. Tibshirani, 1990. Generalized Additive Models. Chapman & Hall, London.
Hering, D., A. Schmidt-Kloiber, J. Murphy, S. Lucke, C. Zamora-Muñoz, M. J. López-Rodríguez, T. Huber & W. Graf, 2009. Potential impact of climate change on aquatic insects: a sensitivity analysis for European caddisflies (Trichoptera) based on distribution patterns and ecological preferences. Aquatic Sciences 71: 3–14.
Herzig, A., 1983. The ecological significance of the relationship between temperature and duration of embryonic development in planktonic freshwater copepods. Hydrobiologia 100: 65–91.
Hirche, H. J., U. Meyer & B. Niehoff, 1997. Egg production of Calanus finmarchicus: effect of temperature, food and season. Marine Biology 127: 609–620.
Huntley, M. & M. D. G. López, 1992. Temperature-dependent production of marine copepods: a global synthesis. American Naturalist 140: 201–242.
Ianora, A., 1998. Copepod life history traits in subtemperate regions. Journal of Marine Systems 15: 337–349.
Jamieson, C. & C. Burns, 1988. The effects of temperature and food on copepodite development, growth and reproduction in three species of Boeckella (Copepoda: Calanoida). Hydrobiologia 164: 235–257.
Jamieson, C. D. & B. Santer, 2003. Maternal aging in the univoltine freshwater copepod Cyclops kolensis: variation in egg sizes, egg development times, and naupliar development times. Hydrobiologia 510: 75–81.
Jeppesen, E., et al., 2011. Zooplankton as indicators in lakes: a scientific-based plea for including zooplankton in the ecological quality assessment of lakes according to the European Water Framework Directive (WFD). Hydrobiologia 676: 279–297. doi:10.1007/s10750-011-0831-0.
Jiménez-Melero, R., 2007. Population dynamics, demography and production of Arctodiaptomus salinus (Copepoda: Calanoida) in a saline endorheic pond. PhD thesis, Universidad de Jaén.
Jiménez-Melero, R., B. Santer & F. Guerrero, 2005. Embryonic and naupliar development of Eudiaptomus gracilis and Eudiaptomus graciloides at different temperatures: comments on individual variability. Journal of Plankton Research 27: 1175–1187.
Jiménez-Melero, R., G. Parra, S. Souissi & F. Guerrero, 2007. Post-embryonic developmental plasticity of Arctodiaptomus salinus (Copepoda: Calanoida) at different temperatures. Journal of Plankton Research 29: 553–567.
Klein Breteler, W. C. M., N. Schogt & J. van der Meer, 1994. The duration of copepod life stages estimated from stage-frequency data. Journal of Plankton Research 16: 1039–1057.
Landry, M. R., 1975. The relationship between temperature and the development of life stages of the marine copepod Acartia clausi Giesbr. Limnology and Oceanography 20: 854–858.
Lapesa, S., T. W. Snell, D. M. Fields & M. Serra, 2004. Selective feeding of Arctodiaptomus salinus (Copepoda, Calanoida) on co-occurring sibling rotifer species. Freshwater Biology 49: 1053–1061.
Lee, H. W., S. Ban, T. Ikeda & T. Matsuishi, 2003. Effect of temperature on development, growth and reproduction in the marine copepod Pseudocalanus newmani at satiating food condition. Journal of Plankton Research 25: 261–271.
López, M. D. G., 1991. Molting and mortality depend on age and stage in naupliar Calanus pacificus: implication for development time of field cohort. Marine Ecology-Progress Series 75: 79–89.
López-González, P. J., F. Guerrero & M. C. Castro, 1998. Seasonal fluctuations in the plankton community in a hypersaline temporary lake (Honda, southern Spain). International Journal of Salt Lake Research 6: 353–371.
Margalef, R., 1983. Limnología. Omega, Barcelona.
Martynova, D. M., N. A. Kazus, U. V. Bathmann, M. Graeve & A. A. Sukhotin, 2011. Seasonal abundance and feeding patterns of copepods Temora longicornis, Centropages hamatus and Acartia spp. in the White Sea (66°N). Polar Biology 34: 1175–1195.
Mazzocchi, M. G. & G. A. Paffenhöfer, 1998. First observations on the biology of Clausocalanus furcatus (Copepoda, Calanoida). Journal of Plankton Research 20: 331–342.
McLaren, I. A., C. J. Corkett & E. J. Zillioux, 1969. Temperature adaptations of copepod eggs from the arctic to the tropics. Biological Bulletin 137: 486–493.
Miracle, M. R., 1976. Distribución en el espacio y en el tiempo de las especies del zooplancton del lago de Banyoles. Ministerio de Agricultura. Instituto Nacional para la Conservación de la Naturaleza, Madrid.
Munch, S. B. & S. Salinas, 2009. Latitudinal variation in lifespan within species is explained by the metabolic theory of ecology. Proceedings of the National Academy of Sciences of the United States of America 106: 13860–13864.
Munro, I. G., 1974. The effect of temperature on the development of egg, naupliar and copepodite stages of two species of copepods, Cyclops vicinus Uljanin and Eudiaptomus gracilis Sars. Oecologia 16: 355–367.
Niehoff, B., 2004. The effect of food limitation on gonad development and egg production of the planktonic copepod Calanus finmarchicus. Journal of Experimental Marine Biology and Ecology 307: 237–259.
Pfister, C. A. & F. R. Stevens, 2002. The genesis of size variability in plants and animals. Ecology 83: 59–72.
Riccardi, N. & M. Mangoni, 1999. Considerations on the biochemical composition of some freshwater zooplankton species. Journal of Limnology 58: 58–65.
Rodríguez, V., F. Guerrero & B. Bautista, 1995. Egg production of individual copepods of Acartia grani Sars from coastal waters: seasonal and diel variability. Journal of Plankton Research 17: 2233–2250.
Rokneddine, A., 2004a. Influence de la salinité et de la température sur la croissance d’Arctodiaptomus salinus (Daday, 1885) (Copepoda, Calanoida), du marais temporaire salé, “La Sebkha Zima”, Maroc. Crustaceana 77: 1025–1044.
Rokneddine, A., 2004b. Influence de la salinité et de la température sur la reproduction d’Arctodiaptomus salinus (Daday, 1885) (Copepoda, Calanoida), du marais temporaire salé, “La Sebkha Zima” (Maroc). Crustaceana 77: 923–940.
Sánchez, N., H. E. Humberto & J. L. Iriarte, 2011. Trophic interactions of pelagic crustaceans in Comau Fjord (Chile): their role in the food web structure. Journal of Plankton Research 33: 1212–1229.
Souissi, S. & S. Ban, 2001. The consequences of individual variability in moulting probability and the aggregation of stages for modelling copepod population dynamics. Journal of Plankton Research 23: 1279–1296.
Tolomeyev, A. P., 2002. Phytoplankton diet of Arctodiaptomus salinus (Copepoda, Calanoida) in Lake Shira (Khakasia). Aquatic Ecology 36: 229–234.
Twombly, S. & N. Tisch, 2002. Fitness consequences of the timing of metamorphosis in a freshwater crustacean. Oikos 97: 213–222.
Uye, S., 1981. Fecundity studies of neritic calanoid copepods Acartia clausi Giesbrecht and A. steueri Smirnov: a simple empirical model of daily egg production. Journal of Experimental Marine Biology and Ecology 50: 255–271.
Valenzano, D. R., E. Terzibasi, A. Cattaneo, L. Domenici & A. Cellerino, 2006. Temperature affects longevity and age-related locomotor and cognitive decay in the short-lived fish Nothobranchius furzeri. Aging Cell 5: 275–278.
Vijverberg, J., 1989. Culture techniques for studies on the growth, development and reproduction of copepods and cladocerans under laboratory and in situ conditions: a review. Freshwater Biology 21: 317–373.
Zotina, T. A., A. P. Tolomeyev & N. N. Degermendzhy, 1999. Lake Shira, a Siberian salt lake: ecosystem structure and function. 1. Major physico-chemical and biological features. International Journal of Salt Lake Research 8: 211–232.
Acknowledgments
This work was supported by the Comisión Interministerial de Ciencia y Tecnología, Spain (CICYT Project PB98-0307). R.J.-M. was supported by a grant from the Spanish Ministry of Science and Technology. Thanks to Consejería de Medio Ambiente for permissions to sample at Laguna Honda. We thank Mike Epps for the English corrections.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Mariana Meerhoff
Rights and permissions
About this article
Cite this article
Jiménez-Melero, R., Parra, G. & Guerrero, F. Effect of temperature, food and individual variability on the embryonic development time and fecundity of Arctodiaptomus salinus (Copepoda: Calanoida) from a shallow saline pond. Hydrobiologia 686, 241–256 (2012). https://doi.org/10.1007/s10750-012-1014-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-012-1014-3