Abstract
The impact of SARS-CoV-2 infection on heart transplant recipients is unknown. Literature is limited to case reports and series. The purpose of this study is to identify the clinical features, outcomes, and immunosuppression strategies of heart transplant recipients with COVID-19 infection. A systematic review was conducted using the search term “Coronavirus” or COVID,” “SARS-CoV-2,” “cardiac transplantation,” and “heart transplant.” Case reports and retrospective studies were gathered by searching Medline/PubMed, Google Scholar, CINAHL, Cochrane CENTRAL, and Web of Science. Thirty-three articles were selected for review. We identified 74 cases of SARS-CoV-2 infection in heart transplant and heart-kidney transplant recipients. The mean age was 60.5 ± 15.8 years, and 82.4% were males with median time from transplant of 6.5 years. Commonest symptoms were fever, cough, and dyspnea, but new left ventricular (LV) dysfunction was rare. Leukocytosis, lymphopenia, elevated inflammatory markers, and bilateral ground-glass opacities were common. Mortality was high, with particularly poor survival in patients who required intensive care unit (ICU) admission and older patients. Immunosuppression involved discontinuation of antimetabolites and steroids. COVID-19 infection in heart transplant (HT) recipients presents similarly to the general population, but new onset of LV dysfunction is uncommon. Immunosuppression strategies include increase in corticosteroids and discontinuation of antimetabolites.
Similar content being viewed by others
Introduction
The first case of coronavirus disease 2019 (COVID-19) was diagnosed in December 2019 in Wuhan, China. This novel infectious disease, which is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has grown into a pandemic with devastating consequences globally [1]. There is a wide variance in clinical manifestations of the disease ranging from asymptomatic, mild upper respiratory symptoms to acute respiratory distress syndrome, multiorgan failure, and death.
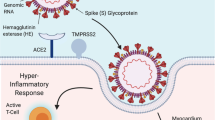
Solid organ transplant recipients are perceived to be at greater risk for severe COVID-19 infection because of their chronically immunosuppressed state. However, there are suggestions that severe COVID-19 results from a systemic hyper-inflammatory state and that immunosuppressive therapy may even be beneficial in selected cases as it may mitigate systemic inflammation [2].
Previous reports analyzed all solid organ transplant recipients with COVID-19 together without making any distinction as to which organ transplant was received by the patient [3]. Recipients of other organs may have different clinical course and outcomes than heart transplant (HT) and heart-kidney transplant (HKT) recipients based on differential survival or immunosuppression strategies. Existing single-center reports on SARS-CoV-2 infection tend to lump all the patients and do not account for individual patient differences, neither do they provide granular data on the individual immunosuppression strategies employed [4]. Cardiac damage of varying severity, from mild troponin elevation to fulminant myocarditis, is not uncommon in COVID-19, but it is unclear how these manifest in the transplanted heart.
Lack of a clear strategy for immunosuppression management in HT and HKT recipients infected with SARS-CoV-2 may predispose them to a worse outcome. Optimal management of immunosuppression in HT recipients infected with SARS-CoV-2 remains unclear.
To address some of these concerns, we analyzed the literature on COVID-19 and systematically reviewed the case reports and series where individual patient data were presented. Papers were limited to 2020 and 2021 to reflect the changes in patterns of management during the pandemic, geography heterogeneity with subsequent differentials in disease and immunosuppression management strategies, and which could reflect more of a real-world experience than being limited to single-center registries.
Our objectives were to include a broader number of HT recipients to better characterize their clinical characteristics, modes of presentation, and, importantly, immunosuppression strategies and clinical outcomes.
Materials and methods
Protocol and registration
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist was adhered to for this systematic review [5]. The protocol was not registered.
Eligibility criteria
Inclusion criteria
Case reports and case review articles that reported on HT and HKT recipients in association with COVID-19 were included.
Exclusion criteria
Studies were excluded if (1) articles were not case reports, case series of observational studies; (2) articles were reviews or editorials; or (3) articles were single-center case series without individual-level patient data. Only articles in the English language were selected.
Information sources and search strategies
A comprehensive literature search was completed using Medline/PubMed, Google Scholar, CINAHL, Cochrane CENTRAL, and Web of Science databases up to and including 27 August 2021, using the terms “Coronavirus,” COVID-19, SARS-CoV-2 in combination with “cardiac transplantation,” “heart transplant,” and “heart-kidney transplant.” We analyzed the literature on COVID-19 (from January 1, 2020, to August 27, 2021) and systematically reviewed the case reports and series with complete individual patient data were presented. Patient demographics, clinical characteristics, laboratory data, management of immunosuppression, and treatment were collected. Outcomes were recorded for all patients. Categorical variables are presented as counts with percentages.
Study selection
Articles were triaged based on whether titles or abstracts met the inclusion criteria. Full-text articles were then read, and those that did not satisfy the inclusion criteria or fit exclusion criteria were excluded. After removing publications that met the exclusion criteria, the remaining publications were further screened for inclusion and exclusion criteria by reading the full-text publications.
Data collection process and data items
Data extracted from articles included the name of first author, year and country of publication, and study design. Patient variables including age, sex, duration of transplant, and presenting complaints on admission were obtained from all studies. Laboratory tests and diagnostic studies, as well as general and immunosuppression management strategies and patient outcomes, were obtained.
Synthesis of results and summary of measures
Information was assessed directly from the case articles. Data were tabulated, evaluated, and summarized.
Risk of bias of the included studies
Potential biases of the included studies were analyzed utilizing the study characteristics. The first author evaluated the methodological quality of the eligible studies. The Joanna Briggs Institute critical appraisal tool for case reports was selected for use in this systematic review [6]. The presence of bias was determined for each article using a checklist the eight questions included in Table 1. The articles received scores to indicate their degree of biases; low (included), high (excluded), or uncertain (more information is required). For this study, if “yes” was answered for half or more of the eight questions on the checklist, the study was at low risk of bias. Similarly, an answer of “no” to half or more of the eight questions meant the study was determined to be at high risk of bias, whereas “unclear” answers were equal to or greater than 50% response.
Results
Study selection
Five databases were used to find 2818 articles related to COVID-19 infection in HT and heart-kidney transplant recipients. Thirty-three studies were then deemed eligible for inclusion in this review [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39]. A PRISMA flow diagram detailing the process of identification, inclusion, and exclusion of studies is shown in Fig. 1.
Study characteristics
Of the thirty-three studies, eleven were retrospective case series [7, 11, 14, 19, 20, 26, 29,30,31,32,33, 38, 39], while twenty-two were case reports [8,9,10, 12, 13, 15,16,17,18, 21,22,23,24,25, 27, 28, 34,35,36,37]. All were peer-reviewed. All articles were published in 2020 and 2021. The total number of patients included in the review is 74.
Risk of bias within the studies
In comparison of the case reports, all the articles were determined to have a very low risk of bias. All the retrospective studies were rated as having low risk of bias. One case report had an intermediate risk of bias [13]. These results are included in Table 1.
Results of individual studies
Categorical variables were described as number (%) and continuous variables were described with mean ± standard deviation or median (IQR), as appropriate.
The search identified 2818 publications. After removing duplicates and screening for exclusion and inclusion criteria, 33 publications were included in the analysis (Fig. 1). A summary of findings from all studies is presented in Table 2.
Synthesis of results
Patient profiles
We identified 74 cases of SARS-CoV-2 infection in heart transplant recipients with data available on individual patients. The mean age was 60.5 ± 15.8 years, and 82.4% were males.
The reports came from 9 countries, including 27 (36.4%) from North America, 27 (36.4%) from Europe, 18 (24.3%) from South America, and 2 (2.7%) from Asia. In total, 68 (91.8%) were HT recipients, while 6 (8.1%) were HKT recipients and 55 (74.3%) were > 1-year post-transplant. The youngest HT recipient infected with COVID-19 who deteriorated and underwent retransplantation was a 22-year-old woman in France [21].
Patients were identified based on testing positive for COVID-19, having a prior HT or HKT. Immunosuppression was assessed based on (1) background immunosuppression; (2) changes in immunosuppression while admitted and then selected clinical outcomes were evaluated.
Presenting complaints
The median time from symptom onset to presentation was 3.5 (2.75–7) days, and fever (59.4%) and cough (59.4%) were the most prevalent symptoms, followed by dyspnea (47.2%), diarrhea (33.8%), and myalgia (25.6%). Fatigue (12.2%), anosmia (12.2%), anorexia (9.4%), and rigors (8.1%) were less common. Bilateral pulmonary infiltrates were seen on chest X-ray in 44 (59.4%) of patients, absent or not, reported in the rest.
The mean temperature recorded was 38.2 ± 0.8 °C, and the mean blood pressure was 123/79 ± 15.6/11.1 mmHg. The distribution of presenting complaints and associated symptoms is found in Table 3.
Past medical history
Of the cohort with fully reported comorbidities, the distribution of comorbidities was as expected from a post-transplant population. Hypertension was reported in 50%, diabetes mellitus in 36.4%, and chronic kidney disease in 31.1% patients and there was significant overlap of these comorbidities in the same patients. The list of comorbidities is also found in Table 3.
Laboratory tests
A summary of laboratory tests is found in Table 4. Mean white blood cell (WBC) count was normal at 6027 ± 3383 cells/mm3, and C-reactive protein (CRP) levels were increased at 42.6 ± 48.2 mg/L. Oxygen saturation and arterial blood gas was unavailable for most studies. Some studies reported either increased troponin-T [7, 9, 14, 19, 24, 25, 27] and/or NT-pro brain natriuretic peptide (NTproBNP) [11, 25].
Diagnostic studies
A list of the diagnostic tests and imaging techniques utilized in the studies is provided in Table 5. All patients were confirmed COVID-19 positive. In patients who underwent chest imaging with computed tomography (CT) scans or chest X-rays (CXR), bilateral infiltrates and ground-glass opacities were discovered in 59.4% of patients.
In five of the studies, 2-D echocardiography revealed decreased LV ejection fraction (EF) [11, 14, 24, 28, 32]. Of the five studies, one did not undergo repeat endomyocardial biopsies due to a recent negative biopsy and the other who had severe biventricular dysfunction that required retransplantation had an endomyocardial biopsy that ruled out acute humoral rejection and no mention was made of viral particles within the myocardium or inflammation [21]. The last case [28] had no evidence of acute cellular rejection (grade 0R) or antibody-mediated rejection and ejection fraction and recovered after 4 days spontaneously.
General management strategies
In terms of anti-inflammatory, antiviral therapies, and immunomodulators, steroids were used most (39.2%), hydroxychloroquine was given to 37.8% and remdesivir to 9.4%, and other anti-viral agents such as lopinavir/ritonavir (5.4%), favipiravir (1.3%), and ganciclovir (2.7%) were rarely used. The use of interleukin 6 (IL-6 inhibitors) mainly tocilizumab and clazakizumab was infrequent occurring in only 9.4% of the cohort. The utilization of antibiotics was common in 25.6% of patients.
The use of vasopressors, human immunoglobulin, renal replacement therapy (excluding long term dialysis) (4%), and venoarterial extracorporeal membrane oxygenation (VA ECMO) (2.7%) was rare. An outline of the management and immunosuppressive therapies used is outlined in Table 6.
Immunosuppressive management strategies
In a majority of patients, 40 (54.1%) were on triple regimen of immunosuppression with either an anti-metabolite (mycophenolate mofetil (MMF), mycophenolic acid (MPA) or azathioprine), calcineurin inhibitor (CNI) (tacrolimus or cyclosporine), steroid, or mammalian target of rapamycin inhibitor (mTORi) (sirolimus or everolimus). Single agent for immunosuppression was rare. Of the patients on triple regimen, the commonest combination was an anti-metabolite, CNI, and steroid combination (48.6%) of the patient.
A 2-drug immunosuppression regimen of anti-metabolite and CNI was seen in 59.4% of patients.
The commonest strategy of managing immunosuppression while admitted was discontinuing or reducing the dose of the anti-metabolite in 36 out of the 63 patients (57.1%) on anti-metabolites. Anti-metabolite dose was reduced in 12 of the 63 patients (19%) and held while admitted in 24 of the 36 patients (38.1%). There was no change in immunosuppression in 20 of the 74 patients (27%) on immunosuppression. The CNI was decreased or stopped at some point in the management course in 11 out of the 64 patients (17.4%) on CNI.
Outcomes
Patient outcomes were included in most of the case reports and case series. Among cases with fully reported data, the median length of stay was 13 days.
Most patients (n = 68, 91.8%) were hospitalized and 5 (6.7%) were categorized as having mild COVID-19 and treated as outpatients. Three patients (4.1%) remained inpatient with uncertain outcome at the time of publication.
Fourteen deaths were reported—two patients died from progressive ARDS and vasoplegic shock that progressed to multiorgan system failure; the third patient had a significant history of multiple cellular and humoral rejection, presented with cardiac arrest, and died. The fourth patient had a hospital course complicated by ischemic cerebrovascular accident and died. Other listed causes of death were septic shock, cardiogenic shock and acute respiratory failure, cardiac arrest, and multi-system organ failure. Two patients were inpatient at the time of case publication [19], while 55 patients were discharged home. Twelve patients required ICU admission (16.2%) of the cohort. Survival in hospitalized patients was 80.2%, while that in patients admitted to the ICU was very poor at 25%.
Outcomes were known for 69 patients: 55 survived, 14 died (mortality was 20.2%).
Risk of bias across the studies
Due to the nature of the descriptive studies, the results presented are liable to investigator bias, selection procedure bias, and selection bias.
Limitation of the study
Most of the reports were observational in nature. Statistical analyses were not performed as there were no control/comparison groups in the included studies. All the desired datasets were not available in all the reports and case series. The therapies for COVID-19 were also changing rapidly during the first 9 months of the pandemic and could have impacted management patterns and outcomes.
Discussion
Outcomes
Our review involves the first 20 months of the COVID-19 pandemic, geography heterogeneity with underlying differentials of disease management, which could reflect more of a real-world experience than being limited to single-center registries.
We found that SARS-CoV-2 infection in HT and HKT recipients in our cohort had a worse mortality (20.2%) than SARS-CoV-2 infection in the general US population [40]. Pulmonary involvement and severe infection correlated with the need for ICU admission and portended a low risk of survival. Despite being immunosuppressed, 80.8% of patients with at least moderately severe disease requiring hospitalization survived SARS-CoV-2 infection.
Bottio et al. [4] in the Northern Italy series and Granger et al. [41] from France have recently published multi-center registries documenting outcomes of HT recipients with SARS-CoV-2 infection. Older age, diabetes mellitus, peripheral vascular disease, previous percutaneous coronary intervention, lower eGFR, and higher NYHA functional class were significantly associated with in-hospital mortality and immunosuppressive drugs were reduced in all hospitalized patients. The French series [41] observed an overall 56% excess year over year (from March–June 2019 to March–June 2020) excess mortality during the early outbreak of COVID among HT recipients. Diabetes mellitus or chronic kidney disease stage ≥ III was associated with greater risk of mortality.
A propensity score-matched analysis of mortality in solid organ transplants with COVID-19 found that older age, higher CRP, and serum creatinine levels were associated with higher mortality but the observed mortality and clinical outcomes were similar in transplant recipients to those seen in the non-transplant population [42]. While most of the therapies were considered experimental in the early days of the pandemic, randomized data has subsequently confirmed the efficacy of intravenous steroids [43], tocilizumab [44], and remdesivir [45] and the inefficacy of hydroxychloroquine [46, 47].
Importantly, left ventricular (LV) systolic dysfunction was a presentation in 6.7% of patients and reassuringly rejection was not seen in any of the cases, and this likely represents COVID myocarditis.
It is important to compare our findings with other similar analyses earlier during the pandemic. Latif et al. [48] in New York had a report on HT recipients with COVID-19 within the first 6 weeks of the pandemic. Most of the patients were admitted for and (25%) required mechanical ventilation. Most (76%) had evidence of myocardial injury and elevated inflammatory biomarkers but none reported cardiac allograft or LV dysfunction. Among patients managed at the study institution, mycophenolate mofetil was discontinued in most patients (70%), and only a minority (26%) had a reduction in the dose of their calcineurin inhibitor. Iacovoni et al. [49] had a similar report in Italy on HT recipients with COVID-19 within the first 4 weeks of the pandemic and found that seven out of 26 (27%) admitted patients died, while 17 (65%) were admitted. Discontinuation of immunosuppression was associated with death (71 vs. 21%, p = 0.02) and patients who died were older than survivors, had a longer time from transplant, and a worse clinical presentation at diagnosis.
Implications for immunosuppression strategies
In our case series, the reduction or cessation of anti-metabolites and use of corticosteroids were a commonly used strategy. Calcineurin inhibitors (CNI) were reduced in a minority of patients. Despite the potential for mTOR inhibitors to worsen pneumonia, it was only held in two out of five patients that were on it prior to admission.
Practical consideration exists in management of immunosuppression in immunosuppressed HT and HKT recipients.
Firstly, severe immunosuppression can lead to greater propensity to infection and worse outcomes. Holding immunosuppressants can alleviate leucopenia and lymphopenia and allow the body to mount a more robust immune response.
Secondly, the cytokine storm syndrome associated with SARS-CoV-2 infection is associated with higher levels of inflammatory markers. Attenuation of the inflammation with corticosteroids with reduction of inflammatory markers can lessen the morbidity from SARS-CoV-2 infection.
Another third consideration will be the duration from time of transplant and history of rejection. There may be less flexibility with reduction of baseline immunosuppression in patients who are less than 1 year from HT with significant rejection history. Sperry et al. [22] reported on the potential for cell-free DNA to assess for allograft injury and inflammatory markers, and to guide further decisions on adjustment of immunosuppression therapy.
Although most programs maintained CNI at therapeutic levels given the known risk of rejection from CNI withdrawal, it is also important to note that protease inhibitors such as ritonavir are potent inhibitors of the metabolism of immunosuppressive drugs including CNI and must be used with caution in transplant patients [50]. CNI are known to affect kidney function; hence, close monitoring is imperative when given concomitantly with lopinavir/ritonavir, especially in light of observed acute kidney injury in COVID-19 patients and adverse effects on mortality [51].
Pneumonitis, a well-known side effect, should prompt the cessation of mTORi in patients with pneumonia or progressing to ARDS. Additionally, the metabolism of mTORi and CNIs may be altered by anti-retroviral agents, hydroxychloroquine, and IL-6 inhibitors, necessitating dose reductions and drug level monitoring.
Although increasing corticosteroids and discontinued antimetabolites is a strategy for immunosuppression in HT patients with COVID-19 infection, the current evidence is anecdotal only. A significant drawback of this strategy is the possibility of developing allograft rejection.
Summarily, our immunosuppressive strategies are in line with the International Society of Heart and Lung Transplant (ISHLT) recommendations which recommend considering holding mycophenolate mofetil, mTOR inhibitors, or azathioprine while admitted with moderate/severe illness [52], although the guidelines were developed after some of the cases were reported. Common drug-drug interactions exist between medications used to treat COVID-19 and transplant medications.
An important direction for designing future studies of SARS-CoV-2 infection in organ transplant recipients should include investigating the role of monoclonal antibodies to COVID-19 which may play a significant role in outcomes for HT patients with COVID-19.
Conclusion
Heart transplant (HT) recipients and heart-kidney (HKT) recipients with COVID-19 infection have a worse survival when compared to the general population, but a new onset of LV dysfunction is rare and needs to be differentiated from acute rejection. Most used alterations to immunosuppression strategies include corticosteroids and discontinuation of antimetabolites. Further research is needed to advance our understanding of COVID-19 infection in heart transplant and heart-kidney transplant recipients especially with regard to the use of monoclonal antibodies for COVID-19.
References
Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y et al (2020) Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 382(13):1199–1207
Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ (2020) COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395(10229):1033–1034
Hoek RAS, Manintveld OC, Betjes MGH, Hellemons ME, Seghers L, Van Kampen JAA et al (2020) COVID-19 in solid organ transplant recipients: a single-center experience. Transpl Int 33(9):1099–1105
Bottio T, Bagozzi L, Fiocco A, Nadali M, Caraffa R, Bifulco O et al (2021) COVID-19 in heart transplant recipients: a multicenter analysis of the Northern Italian outbreak. JACC Heart Fail 9(1):52–61
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
The Joanna Briggs Institute (2017) Checklist for Case Reports. https://jbi.global/sites/default/files/2019-05/JBI_Critical_Appraisal-Checklist_for_Case_Reports2017_0.pdf. Cited 15 Feb 2021
Holzhauser L, Lourenco L, Sarswat N, Kim G, Chung B, Nguyen AB (2020) Early experience of COVID-19 in 2 heart transplant recipients: case reports and review of treatment options. Am J Transplant 20(10):2916–2922
Mattioli M, Fustini E, Gennarini S (2020) Heart transplant recipient patient with COVID-19 treated with tocilizumab. Transpl Infect Dis 22(6):e13380
Mathies D, Rauschning D, Wagner U, Mueller F, Maibaum M, Binnemann C et al (2020) A case of SARS-CoV-2 pneumonia with successful antiviral therapy in a 77-year-old man with a heart transplant. Am J Transplant 20(7):1925–1929
Kates OS, Fisher CE, Stankiewicz-Karita HC, Shepherd AK, Church EC, Kapnadak SG et al (2020) Earliest cases of coronavirus disease 2019 (COVID-19) identified in solid organ transplant recipients in the United States. Am J Transplant 20(7):1885–1890
Ahluwalia M, Givertz MM, Mehra MR (2020) A proposed strategy for management of immunosuppression in heart transplant patients with COVID-19. Clin Transplant 34(11):e14032
Ammirati E, Travi G, Orcese C, Sacco A, Auricchio S, Frigerio M et al (2020) Heart-kidney transplanted patient affected by COVID-19 pneumonia treated with tocilizumab on top of immunosuppressive maintenance therapy. Int J Cardiol Heart Vasc 29:100596
Bösch F, Börner N, Kemmner S, Lampert C, Jacob S, Koliogiannis D et al (2020) Attenuated early inflammatory response in solid organ recipients with COVID-19. Clin Transplant 34(10):e14027
Caraffa R, Bagozzi L, Fiocco A, Bifulco O, Nadali M, Ponzoni M et al (2020) Coronavirus disease 2019 (COVID-19) in the heart transplant population: a single-centre experience. Eur J Cardiothorac Surg 58(5):899–906
Decker A, Welzel M, Laubner K, Grundmann S, Kochs G, Panning M et al (2020) Prolonged SARS-CoV-2 shedding and mild course of COVID-19 in a patient after recent heart transplantation. Am J Transplant 20(11):3239–3245
Fung M, Chiu CY, DeVoe C, Doernberg SB, Schwartz BS, Langelier C et al (2020) Clinical outcomes and serologic response in solid organ transplant recipients with COVID-19: a case series from the United States. Am J Transplant 20(11):3225–3233
Jang K, Khatri A, Majure DT (2020) COVID-19 leading to acute encephalopathy in a patient with heart transplant. J Heart Lung Transplant 39(8):853–855
Kadosh BS, Pavone J, Wu M, Reyentovich A, Gidea C (2020) Collapsing glomerulopathy associated with COVID-19 infection in a heart transplant recipient. J Heart Lung Transplant 39(8):855–857
Lima B, Gibson GT, Vullaganti S, Malhame K, Maybaum S, Hussain ST et al (2020) COVID-19 in recent heart transplant recipients: clinicopathologic features and early outcomes. Transpl Infect Dis 22(5):e13382
Schtruk LE, Miranda J, Salles V, Sales A, Lobbe L, Cavalcante V et al (2020) COVID-19 infection in heart transplantation: case reports. Arq Bras Cardiol 115(3):574–578
Soquet J, Rousse N, Moussa M, Goeminne C, Deblauwe D, Vuotto F et al (2020) Heart retransplantation following COVID-19 illness in a heart transplant recipient. J Heart Lung Transplant 39(9):983–985
Sperry BW, Khumri TM, Kao AC (2020) Donor-derived cell-free DNA in a heart transplant patient with COVID-19. Clin Transplant 34(11):e14070
Vaidya G, Czer LSC, Kobashigawa J, Kittleson M, Patel J, Chang D et al (2020) Successful treatment of severe COVID-19 pneumonia with clazakizumab in a heart transplant recipient: a case report. Transplant Proc 52(9):2711–2714
Vilaro J, Al-Ani M, Manjarres DG, Lascano JE, Cherabuddi K, Elgendy AY et al (2020) Severe COVID-19 after recent heart transplantation complicated by allograft dysfunction. JACC Case Rep 2(9):1347–1350
Fried JA, Ramasubbu K, Bhatt R, Topkara VK, Clerkin KJ, Horn E et al (2020) The variety of cardiovascular presentations of COVID-19. Circulation 141(23):1930–1936
Li F, Cai J, Dong N (2020) First cases of COVID-19 in heart transplantation from China. J Heart Lung Transplant 39(5):496–497
Hsu JJ, Gaynor P, Kamath M, Fan A, Al-Saffar F, Cruz D et al (2020) COVID-19 in a high-risk dual heart and kidney transplant recipient. Am J Transplant 20(7):1911–1915
Berg N, Ilonze O, Bajpai V, Guglin M, Rao R (2021) Acute biventricular heart failure after COVID-19 infection in an orthotropic heart transplant patient: a case report. Transplant Proc 53(4):1224–1226
Ballout JA, Ahmed T, Kolodziej AR (2021) COVID-19 and heart transplant: a case series and review of the literature. Transplant Proc 53(4):1219–1223
Felldin M, Softeland JM, Magnusson J, Ekberg J, Karason K, Schult A et al (2021) Initial report From a Swedish high-volume transplant center after the first wave of the COVID-19 pandemic. Transplantation 105(1):108–114
Fernandez-Ruiz M, Andres A, Loinaz C, Delgado JF, Lopez-Medrano F, San Juan R et al (2020) COVID-19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transplant 20(7):1849–1858
Gozzi-Silva SC, Benard G, Alberca RW, Yendo TM, Teixeira FME, Oliveira LM et al (2021) SARS-CoV-2 infection and CMV dissemination in transplant recipients as a treatment for chagas cardiomyopathy: a case report. Trop Med Infect Dis 6(1):22
Guerreiro GP, Silveira L, Manuel V, Steffen SP, Bacal F, Gaiotto FA et al (2021) COVID-19 in early postoperative heart transplantation - initial experience. Arq Bras Cardiol 116(1):144–146
Isik ME, Gunay D, Aksut M, Menekse S, Uygun-Kizmaz Y, Kirali MK (2021) COVID-19 presenting with diarrhea in a heart transplant patient. Turk Gogus Kalp Damar Cerrahisi Derg 29(1):119–121
Mangiameli G, Al Zreibi C, Caudron J, Arame A, Le Pimpec-Barthes F (2020) Unexpected evolution of COVID-19 in a heart transplant patient with multimorbidity recently submitted to thoracic surgery. Minerva Chir 75(6):467–468
Martens T, Hens L, De Pauw M, Van Belleghem Y (2021) Heart transplantation complicated by COVID-19 infection. Ann Thorac Surg
Schreiber A, Elango K, Hong K, Ahsan C (2021) Cardiac transplant recipient with COVID-19 induced acute hypoxic respiratory failure: a case report. Eur Heart J Case Rep 5(6):ytab217
Soriano RVM, Rossi Neto JM, Finger MA, Santos CC (2021) COVID-19 in heart transplant recipients in Sao Paulo: a case series. Arq Bras Cardiol 116(2 suppl 1):1–3
Tchana-Sato V, Ancion A, Tridetti J, Sakalihasan N, Hayette MP, Detry O et al (2021) Clinical course and challenging management of early COVID-19 infection after heart transplantation: case report of two patients. BMC Infect Dis 21(1):89
Bilinski A, Emanuel EJ (2020) COVID-19 and excess all-cause mortality in the US and 18 comparison countries. JAMA 324(20):2100–2102
Granger C, Guedeney P, Arnaud C, Guendouz S, Cimadevilla C, Kerneis M et al (2021) Clinical manifestations and outcomes of coronavirus disease-19 in heart transplant recipients: a multicentre case series with a systematic review and meta-analysis. Transplant Int 34(4):721–731
Linares L, Cofan F, Diekmann F, Herrera S, Marcos MA, Castel MA et al (2021) A propensity score-matched analysis of mortality in solid organ transplant patients with COVID-19 compared to non-solid organ transplant patients. PLoS One 16(3):e0247251
Group RC, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL et al (2021) Dexamethasone in hospitalized patients with COVID-19. New Engl J Med 384(8):693–704
Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhart JD et al (2021) Tocilizumab in patients hospitalized with COVID-19 pneumonia. N Engl J Med 384(1):20–30
Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC et al (2020) Remdesivir for the treatment of COVID-19 - final report. N Engl J Med 383(19):1813–1826
Group RC, Horby P, Mafham M, Linsell L, Bell JL, Staplin N et al (2020) Effect of hydroxychloroquine in hospitalized patients with COVID-19. N Engl J Med 383(21):2030–2040
Mitja O, Corbacho-Monne M, Ubals M, Alemany A, Suner C, Tebe C et al (2021) A Cluster-randomized trial of hydroxychloroquine for prevention of COVID-19. N Engl J Medicine 384(5):417–427
Latif F, Farr MA, Clerkin KJ, Habal MV, Takeda K, Naka Y et al (2020) Characteristics and outcomes of recipients of heart transplant with coronavirus disease 2019. JAMA Cardiol 5(10):1165–1169
Iacovoni A, Boffini M, Pidello S, Simonato E, Barbero C, Sebastiani R et al (2020) A case series of novel coronavirus infection in heart transplantation from 2 centers in the pandemic area in the North of Italy. Journal Heart Lung Transplant 39(10):1081–1088
Jain AB, Venkataramanan R, Eghtesad B, Marcos A, Ragni M, Shapiro R et al (2003) Effect of coadministered lopinavir and ritonavir (Kaletra) on tacrolimus blood concentration in liver transplantation patients. Liver Transpl 9(9):954–960
Bottiger Y, Brattstrom C, Tyden G, Sawe J, Groth CG (1999) Tacrolimus whole blood concentrations correlate closely to side-effects in renal transplant recipients. Br J Clin Pharmacol 48(3):445–448
Aslam S (2021) Guidance from the International Society of Heart and Lung Transplantation regarding the SARS CoV-2 pandemic USA: International Society of Heart and Lung Transplant. https://ishlt.org/ishlt/media/documents/SARS-CoV-2_Guidance-for-Cardiothoracic-Transplant-and-VAD-center.pdf
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ilonze, O.J., Ballut, K., Rao, R.S. et al. SARS-CoV-2 infection in heart transplant recipients: a systematic literature review of clinical outcomes and immunosuppression strategies. Heart Fail Rev 27, 1653–1663 (2022). https://doi.org/10.1007/s10741-021-10181-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-021-10181-y