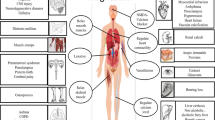
Abstract
Vitamin C (Vit C) is an ideal antioxidant as it is easily available, water soluble, very potent, least toxic, regenerates other antioxidants particularly Vit E, and acts as a cofactor for different enzymes. It has received much attention due to its ability in limiting reactive oxygen species, oxidative stress, and nitrosative stress, as well as it helps to maintain some of the normal metabolic functions of the cell. However, over 140 clinical trials using Vit C in different pathological conditions such as myocardial infarction, gastritis, diabetes, hypertension, stroke, and cancer have yielded inconsistent results. Such a divergence calls for new strategies to establish practical significance of Vit C in heart failure or even in its prevention. For a better understanding of Vit C functioning, it is important to revisit its transport across the cell membrane and subcellular interactions. In this review, we have highlighted some historical details of Vit C and its transporters in the heart with a particular focus on heart failure in cancer chemotherapy.



Similar content being viewed by others
Change history
16 November 2020
A Correction to this paper has been published: https://doi.org/10.1007/s10741-020-10043-z
References
Carr AC, Frei B (1999) Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr 69:1086–1107
Drake VJ, Frei B (2011) In: Herrmann W, Obeid R (eds) Vitamins in the prevention of human diseases. Walter de Gruyter, Berlin, pp 347–362
Cook NR, Albert CM, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, Buring JE, Manson J (2007) A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women - results from the Women’s Antioxidant Cardiovascular Study. Arch Intern Med 167:1610–1618
Galang N, Sasaki H, Maulik N (2000) Apoptotic cell death during ischemia/reperfusion and its attenuation by antioxidant therapy. Toxicology 148:111–118
Hercberg S, Galan P, Preziosi P, Bertrais S, Mennen L, Malvy D, Roussel AM, Favier A, Briançon S (2004) The SU.VI.MAX study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med 164:2335–2242
Blumberg J, Heaney RP, Huncharek M, Scholl T, Stampfer M, Vieth R, Weaver CM, Zeisel SH (2010) Evidence-based criteria in the nutritional context. Nutr Rev 68:478–484
Frei B (2004) Efficacy of dietary antioxidants to prevent oxidative damage and inhibit chronic disease. J Nutr 134:3196S–3198S
Lykkesfeldt J, Poulsen HE (2010) Is vitamin C supplementation beneficial? Lessons learned from randomised controlled trials. Br J Nutr 103:1251–1259
Ludke AR, Sharma AK, Akolkar G, Bajpai G, Singal PK (2012a) Downregulation of vitamin C transporter SVCT-2 in doxorubicin-induced cardiomyocyte injury. Am J Physiol Cell Physiol 303:C645–C653
Ludke A, Sharma AK, Bagchi AK, Singal PK (2012b) Subcellular basis of vitamin C protection against doxorubicin-induced changes in rat cardiomyocytes. Mol Cell Biochem 360:215–224
Léger D (2008) Scurvy: reemergence of nutritional deficiencies. Can Fam Physician 54:1403–1406
Lind J (1753) A treatise of the scurvy, in three parts: containing an inquiry into the nature, causes, and cure, of that disease; together with a critical and chronological view of what has been published on the subject. Royal College of Physicians in Edinburg, London, pp 193–222
Funk C (1912) The etiology of the deficiency diseases. Beri-beri, polyneuritis in birds, epidemic dropsy, scurvy, experimental scurvy in animals, infantile scurvy, ship beri-beri, pellagra. J State Med 20:341–368
Haworth WN, Hirst EL (1933) Synthesis of ascorbic acid. J Soc Chem Ind Lond 52:645–647
Haworth WN, Hirst EL (1937) The Nobel prize in chemistry NobelPrize.org
Carr AC, Vissers MC (2013) Synthetic or food-derived vitamin C - are they equally bioavailable? Nutrients 5:4284–4304
Lohmann W, Winzenburg J (1983) Structure of ascorbic acid and its biological function: V. transport of ascorbate and isoascorbate across artificial membranes as studied by the spin label technique. Z Naturforsch C Biosci 38:923–925
Corti A, Casini AF, Pompella A (2010) Cellular pathways for transport and efflux of ascorbate and dehydroascorbate. Arch Biochem Biophys 500:107–115
Du J, Cullen JJ, Buettner GR (2012) Ascorbic acid: chemistry, biology and the treatment of cancer. Biochim Biophys Acta 1826:443–457
Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y (1999) Criteria and recommendations for vitamin C intake. JAMA 281:1415–1423
National Research Council (US) Subcommittee on the Tenth Edition of the Recommended Dietary Allowances (1989) Recommended dietary allowances, 10th edn. National Academies Press (US), Washington (DC) 1989
German Nutrition Society (DGE) (2015) New reference values for vitamin C intake. Ann Nutr Metab 67:13–20
Capellmann M, Becka M, Bolt HM (1994) A note on distribution of human plasma levels of ascorbic and dehydroascorbic acid. J Physiol Pharmacol 45:183–187
Hornig D (1975) Distribution of ascorbic acid, metabolites and analogues in man and animals. Ann N Y Acad Sci 258:103–118
Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen XZ, Wang Y, Brubaker RF, Hediger MA (1999) A family of mammalian Na-dependent L-ascorbic acid transporters. Nature 399:70–75
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and nitrate in biological fluids. Anal Biochem 126:131–138
Akolkar G, da Silva DD, Ayyappan P, Bagchi AK, Jassal DS, Salemi VMC, Irigoyen MC, De Angelis K, Singal PK (2017a) Vitamin C mitigates oxidative/nitrosative stress and inflammation in doxorubicin-induced cardiomyopathy. Am J Physiol Heart Circ Physiol 313:H795–H809
Akolkar G, Bagchi AK, Ayyappan P, Jassal DS, Singal PK (2017b) Doxorubicin-induced nitrosative stress is mitigated by vitamin C via the modulation of nitric oxide synthases. Am J Physiol Cell Physiol 312:C418–C442
McNulty AL, Vail TP, Kraus VB (2005) Chondrocyte transport and concentration of ascorbic acid is mediated by SVCT2. Biochim Biophys Acta 1712:212–221
Rivas CI, Zuniga FA, Salas-Burgos A, Mardones L, Ormazabal V, Vera JC (2008) Vitamin C transporters. J Physiol Biochem 64:357–375
Savini I, Rossi A, Pierro C, Avigliano L, Catani MV (2008) SVCT1 and SVCT2: key proteins for vitamin C uptake. Amino Acids 34:347–355
Wilson JX (2002) The physiological role of dehydroascorbic acid. FEBS Lett 527:5–9
Daruwala R, Song J, Koh WS, Rumsey SC, Levine M (1999) Cloning and functional characterization of the human sodium-dependent vitamin C transporters hSVCT1 and hSVCT2. FEBS Lett 460:480–484
Rajan DP, Huang W, Dutta B, Devoe LD, Leibach FH, Ganapathy V, Prasad PD (1999) Human placental sodium-dependent vitamin C transporter (SVCT2): molecular cloning and transport function. Biochem Biophys Res Commun 262:762–768
Harrison FE, Dawes SM, Meredith ME, Babaev VR, Li L, May JM (2010a) Low vitamin C and increased oxidative stress and cell death in mice that lack the sodium-dependent vitamin C transporter SVCT2. Free Radic Biol Med 49:821–829
Harrison FE, Green RJ, Dawes SM, May JM (2010b) Vitamin C distribution and retention in the mouse brain. Brain Res 1348:181–186
Sotiriou S, Gispert S, Cheng J, Wang Y, Chen A, Hoogstraten-Miller S, Miller GF, Kwon O, Levine M, Guttentag SH, Nussbaum RL (2002) Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nat Med 8:514–517
Liang WJ, Johnson D, Ma LS, Jarvis SM, Wei-Jun L (2002) Regulation of the human vitamin C transporters expressed in COS-1 cells by protein kinase C. Am J Physiol Cell Physiol 283:C1696–C1704
MacDonald L, Thumser AE, Sharp P (2002) Decreased expression of the vitamin C transporter SVCT1 by ascorbic acid in a human intestinal epithelial cell line. Br J Nutr 87:97–100
Savini I, Catani MV, Arnone R, Rossi A, Frega G, Del Principe D, Avigliano L (2007) Translational control of the ascorbic acid transporter SVCT2 in human platelets. Free Radic Biol Med 42:608–616
Biondi C, Pavan B, Dalpiaz A, Medici S, Lunghi L, Vesce F (2007) Expression and characterization of vitamin C transporter in the human trophoblast cell line HTR-8/SVneo: effect of steroids, flavonoids and NSAIDs. Mol Hum Reprod 13:77–83
Dixon SJ, Wilson JX (1992) Adaptive regulation of ascorbate transport in osteoblastic cells. J Bone Miner Res 7:675–681
Fujita I, Hirano J, Itoh N, Nakanishi T, Tanaka K (2001) Dexamethasone induces sodium-dependent vitamin C transporter in a mouse osteoblastic cell line MC3T3-E1. Br J Nutr 86:145–149
Berger UV, Lu XC, LiuW TZ, Slusher BS, Hediger MA (2003) Effect of middle cerebral artery occlusion on mRNA expression for the sodium-coupled vitamin C transporter SVCT2 in rat brain. J Neurochem 86:896–906
Lutsenko EA, Carcamo JM, Golde DW (2004) A human sodium-dependent vitamin C transporter 2 isoform acts as a dominant-negative inhibitor of ascorbic acid transport. Mol Cell Biol 24:3150–3156
Savini I, Rossi A, Catani MV, Ceci R, Avigliano L (2007b) Redox regulation of vitamin. C transporter SVCT2 in C2C12 myotubes. Biochem Biophys Res Commun 361:385–390
Wilson JX, Jaworski EM, Kulaga A, Dixon SJ (1990) Substrate regulation of ascorbate transport activity in astrocytes. Neurochem Res 15:1037–1043
Vera JC, Rivas CI, Velasquez FV, Zhang RH, Concha II, Golde DW (1995) Resolution of the facilitated transport of dehydroascorbic acid from its intracellular accumulation as ascorbic acid. J Biol Chem 270:23706–23712
Vera JC, Rivas CI, Zhang RH, Farber CM, Golde DW (1994) Human HL-60 myeloid leukemia cells transport dehydroascorbic acid via the glucose transporters and accumulate reduced ascorbic acid. Blood 84:1628–1634
Montel-Hagen A, Blanc L, Boyer-Clavel M, Jacquet C, Vidal M, Sitbon M, Taylor N (2008) The Glut1 and Glut4 glucose transporters are differentially expressed during perinatal and postnatal erythropoiesis. Blood 112:4729–4738
Rumsey S, Levine M (1998) Absorption, transport and disposition of ascorbic acid in humans. J Nutr Biochem 9:116–130
Harrison FE, May JM (2009) Vitamin C function in the brain: vital role of the ascorbate transporter SVCT2. Free Radic Biol Med 46:719–730
Banhegyi G, Benedetti A, Margittai E, Marcolongo P, Fulceri R, Nemeth CE, Szarka A (2014) Subcellular compartmentation of ascorbate and its variation in disease states. Biochim Biophys Acta 1843:1909–1916
May JM (2000) How does ascorbic acid prevent endothelial dysfunction? Free Radic Biol Med 28:1421–1429
Winkler BS, Orselli SM, Rex TS (1994) The redox couple between glutathione and ascorbic acid: a chemical and physiological perspective. Free Radic Biol Med 17:333–349
Halliwell B (1996) Vitamin C: antioxidant or pro-oxidant in vivo. Free Radic Res 25:439–454
Linster CL, Van Schaftingen E (2007) Vitamin C. Biosynthesis, recycling and degradation in mammals. FEBS J 274:1–22
Leonarduzzi G, Sottero B, Poli G (2010) Targeting tissue oxidative damage by means of cell signaling modulators: the antioxidant concept revisited. Pharmacol Ther 128:336–374
Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, Chen S, Corpe C, Dutta A, Dutta SK, Levine M (2003) Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr 22:18–35
Traber MG, Stevens JF (2011) Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radic Biol Med 51:1000–1013
Levine M, Padayatty SJ (2014) Vitamin C. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR (eds) Modern nutrition in health and disease, 11th edn. Lippincott Williams & Wilkins, Philadelphia, pp 399–415
Englard S, Seifter S (1986) The biochemical functions of ascorbic acid. Annu Rev Nutr 6:365–406
De Tullio MC (2012) Beyond the antioxidant: the double life of vitamin C. Subcell Biochem 56:49–65
Knowles J, Raval RR, Harris AL, Ratcliffe PJ (2003) Effect of ascorbate on the activity of hypoxia-inducible factor in cancer cells. Cancer Res 63:1764–1768
Geesin JC, Darr D, Kaufman R, Murad S, Pinnell SR (1988) Ascorbic acid specifically increases type I and type III procollagen messenger RNA levels in human skin fibroblast. J Investig Dermatol 90:420–424
Heller R, Unbehaun A, Schellenberg B, Mayer B, Werner-Felmayer G, Werner ER (2001) L-ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J Biol Chem 276:40–47
Piccirillo G, Nocco M, Moisè A, Lionetti M, Naso C, Carlo SD, Marigliano V (2003) Influence of vitamin C on baroreflex sensitivity in chronic heart failure. Hypertension 41:1240–1245
Harrington D, Caots AJ (1997) Mechanisms of exercise intolerance in congestive heart failure. Curr Opin Cardiol 12:224–232
Chapleau MW, Cunningham T, Sullivan MJ, Watchel RE, Abboud FM (1995) Structural versus functional modulation of the arterial baroreflex. Hypertension 26:341–347
Farbstein D, Kozak-Blickstein A, Levy AP (2010) Antioxidant vitamins and their use in preventing cardiovascular disease. Molecules 15:8098–8110
Ferrari R, Agnoletti L, Comini L, Gaia G, Bachetti T, Cargnoni A, Ceconi C, Curello S, Visioli O (1998) Oxidative stress during myocardial ischemia and heart failure. Eur Heart J 19:B2–B11
Gianni L, Corden BJ, Myers CE (1983) The biochemical basis of anthracycline toxicity and anti-tumor activity. Rev Biochem Toxicol 5:1–82
Kaul N, Siveski-Iliskovic N, Hill M, Slezak J, Singal PK (1993) Free radicals and the heart. J Pharmacol Toxicol Methods 30:55–67
Khaper N, Bryan S, Dhingra S, Singal R, Bajaj A, Pathak CM, Singal PK (2010) Targeting the vicious inflammation-oxidative stress cycle for the management of heart failure. Antioxid Redox Signal 13:1033–1049
Kumar D, Lou H, Singal PK (2002) Oxidative stress and apoptosis in heart dysfunction. Herz 27:662–668
Lou H, Danelisen I, Singal PK (2004) Cytokines are not upregulated in doxorubicin-induced cardiomyopathy and heart failure. J Mol Cell Cardiol 36:683–690
Seddon M, Looi YH, Shah AM (2007) Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart 93:903–907
Singal PK, Khaper N, Farahmand F, Belló-Klein A (2000) Oxidative stress in congestive heart failure. Curr Cardiol Rep 2:206–211
Singal PK, Khaper N, Palace V, Kumar D (1998) The role of oxidative stress in the genesis of heart disease. Cardiovasc Res 40:426–432
Siti HN, Kamisah Y, Kamsiah J (2015) The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vasc Pharmacol 71:40–56
Palace VP, Hill MF, Farahmand F, Singal PK (1999a) Mobilization of antioxidant vitamin pools and hemodynamic function after myocardial infarction. Circulation 99:121–126
Palace V, Kumar D, Hill MF, Khaper N, Singal PK (1999b) Regional differences in non-enzymatic antioxidants in the heart under control and oxidative stress conditions. J Mol Cell Cardiol 31:193–202
Block KI, Koch AC, Mead MN, Tothy PK, Newman RA, Gyllenhaal C (2007) Impact of antioxidant supplementation on chemotherapeutic efficacy: a systematic review of the evidence from randomized controlled trials. Cancer Treat Rev 33:407–418
Kim H, Bae S, Kim Y, Cho CH, Kim SJ, Kim YJ, Lee SP, Kim HR, Hwang YI, Kang SJ, Lee WJ (2013) Vitamin C prevents stress-induced damage on the heart caused by the death of cardiomyocytes, through down-regulation of the excessive production of catecholamine, TNF-alpha, and 157 ROS production in gulo (-/-) vit C-insufficient mice. Free Radic Biol Med 65:573–583
Kupari M, Rapola J (2012) Reversible pulmonary hypertension associated with vitamin C deficiency. Chest 142:225–227
Mayland CR, Bennett MI, Allan K (2005) Vitamin C deficiency in cancer patients. Palliat Med 19:17–20
Moradi-Arzeloo M, Farshid AA, Tamaddonfard E, Asri-Rezaei S (2016) Effects of histidine and vitamin C on isoproterenol-induced acute myocardial infarction in rats. Vet Res Forum 7:47–54
Weijl NI, Hopman GD, Wipkink-Bakker A, Lentjes EG, Berger HM, Cleton FJ, Osanto S (1998) Cisplatin combination chemotherapy induces a fall in plasma antioxidants of cancer patients. Ann Oncol 9:1331–1337
Tan C, Tasaka H, Yu KP, Murphy ML, Karnofsky DA (1967) Daunomycin, an antitumor antibiotic, in the treatment of neoplastic disease. Clinical evaluation with special reference to childhood leukemia. Cancer 20:333–353
Singal PK, Iliskovic N (1998) Doxorubicin-induced cardiomyopathy. N Engl J Med 339:900–905
Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL (2012) Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol 52:1213–1225
Xu MF, Tang PL, Qian ZM, Ashraf M (2001) Effects by doxorubicin on the myocardium are mediated by oxygen free radicals. Life Sci 68:889–901
Singal PK, Deally C, Weinberg LE (1987) Subcellular effects of adriamycin in the heart: a concise review. J Mol Cell Cardiol 19:817–828
Arai M, Yoguchi A, Takizawa T, Yokoyama T, Kanda T, Kurabayashi M, Nagai R (2000) Mechanism of doxorubicin-induced inhibition of sarcoplasmic reticulum Ca(2+)-ATPase gene transcription. Circ Res 86:8–14
Li T, Singal PK (2000) Adriamycin-induced early changes in myocardial antioxidant enzymes and their modulation by probucol. Circulation 102:2105–2110
Lefrak EA, Pitha S, Rosenheim JA, Gottlieb A (1973) A clinico-pathologic analysis of adriamycin cardiotoxicity. Cancer 32:302–314
Goncalves TL, Erthal F, Corte CL, Muller LG, Piovezan CM, Nogueira CW, Rocha JB (2005) Involvement of oxidative stress in the pre-malignant and malignant states of cervical cancer in women. Clin Biochem 38:1071–1075
Mukhopadhyay P, Rajesh M, Batkai S, Kashiwaya Y, Hasko G, Liaudet L, Szabo C, Pacher P (2009) Role of superoxide, nitric oxide, and peroxynitrite in doxorubicin-induced cell death in vivo and in vitro. Am J Physiol Heart Circ Physiol 296:H1466–H1483
Pizarro M, Troncoso R, Martinez GJ, Chiong M, Castro PF, Lavandero S (2016) Basal autophagy protects cardiomyocytes from doxorubicin-induced toxicity. Toxicology 370:41–48
Karajibani M, Hashemi M, Montazerifar F, Dikshit M (2010) Effect of vitamin E and C supplements on antioxidant defense system in cardiovascular disease patients in Zahedan, Southeast Iran. J Nutr Sci Vitaminol 56:436–440
Park JH, Davis KR, Lee G, Jung M, Jung Y, Park J, Yi SY, Lee MA, Lee S, Yeom CH, Kim J (2012) Ascorbic acid alleviates toxicity of paclitaxel without interfering with the anticancer efficacy in mice. Nutr Res 32:873–883
Yen HC, Oberley TD, Gairola CG, Szweda LI, St Clair DK (1999) Manganese superoxide dismutase protects mitochondrial complex I against adriamycin induced cardiomyopathy in transgenic mice. Arch Biochem Biophys 362:59–66
Li T, Danelisen I, Bello-Klein A, Singal PK (2000) Effects of probucol on changes of antioxidant enzymes in adriamycin-induced cardiomyopathy in rats. Cardiovasc Res 46:523–530
Iliskovic N, Hasinoff BB, Malisza KL, Li T, Danelisen I, Singal PK (1999) Mechanisms of beneficial effects of probucol in adriamycin cardiomyopathy. Mol Cell Biochem 196:43–49
Siveski-Iliskovic N, Kaul N, Singal PK (1994) Probucol promotes endogenous antioxidants and provides protection against adriamycin-induced cardiomyopathy in rats. Circulation 89:2829–2835
Fallah-Rad N, Walker JR, Wassef A, Lytwyn M, Bohonis S, Fang T, Tian G, Kirkpatrick ID, Singal PK, Krahn M, Grenier D, Jassal DS (2011) The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. J Am Coll Cardiol 57:2263–2270
Walker JR, Sharma A, Lytwyn M, Bohonis S, Thliveris J, Singal PK, Jassal DS (2011) The cardioprotective role of probucol against anthracycline and trastuzumab-mediated cardiotoxicity. J Am Soc Echocardiogr 24:699–705
Goyal V, Bews H, Cheung D, Premecz S, Mandal S, Shaikh B, Best R, Bhindi R, Chaudhary R, Ravandi A, Thliveris J, Singal PK, Niraula S, Jassal DS (2016) The cardioprotective role of N-acetyl cysteine amide in the prevention of doxorubicin and trastuzumab-mediated cardiac dysfunction. Can J Cardiol 32:1513–1519
Siveski-Iliskovic N, Hill M, Singal PK (1995) Probucol protects against adriamycin cardiomyopathy without interfering with its antitumor effect. Circulation 91:10–15
Buttke TM, Sandstrom PA (1994) Oxidative stress as a mediator of apoptosis. Immunol Today 15:7–10
Kumar D, Kirshenbaum LA, Li T, Danelisen I, Singal PK (2001) Apoptosis in adriamycin cardiomyopathy and its modulation by probucol. Antioxid Redox Signal 3:135–145
Sharma AK, Dhingra S, Khaper N, Singal PK (2007) Activation of apoptotic processes during transition from hypertrophy to heart failure in guinea pigs. Am J Physiol Heart Circ Physiol 293:H1384–H1390
Diaz MN, Frei B, Vita JA, Keaney JF Jr (1997) Antioxidants and atherosclerotic heart disease. N Engl J Med 337:408–416
Hasty AH, Gruen ML, Terry ES, Surmi BK, Atkinson RD, Gao L, Morrow JD (2007) Effects of vitamin E on oxidative stress and atherosclerosis in an obese hyperlipidemic mouse model. J Nutr Biochem 18:127–133
Monastyrskaya E, Folarin N, Malyshev I, Green C, Andreeva L (2002) Application of the nitric oxide donor SNAP to cardiomyocytes in culture provides protection against oxidative stress. Nitric Oxide 7:127–231
Abhilash PA, Harikrishnan R, Indira M (2012) Ascorbic acid supplementation causes faster restoration of reduced glutathione content in the regression of alcohol-induced hepatotoxicity in male guinea pigs. Redox Rep 17:72–79
Addis P, Shecterle LM, Alexander J (2012) Cellular protection during oxidative stress – a potential role for d-ribose and antioxidants. J Diet Suppl 9:178–182
Ignarro LJ (1990) Nitric oxide. A novel signal transduction mechanism for transcellular communication. Hypertension 16:477–483
Cindrova-Davies T, Spasic-Boskovic O, Jauniaux E, Charnock-Jones DS, Burton GJ (2007) Nuclear factor-κB, p38, and stress-activated protein kinase mitogen-activated protein kinase signaling pathways regulate proinflammatory cytokines and apoptosis in human placental explants in response to oxidative stress: effects of antioxidant vitamins. Am J Pathol 170:11–20
Papparella I, Ceolotto G, Berto L, Cavalli M, Bova S, Cargnelli G, Ruga E, Milanesi O, Franco L, Mazzoni M, Petrelli L, Nussdorfer GG, Semplicini A (2007) Vitamin C prevents zidovudine-induced NADPH oxidase activation and hypertension in the rat. Cardiovasc Res 73:432–438
Huang A, Vita JA, Venema RC, Keaney JF Jr (2000) Ascorbic acid enhances endothelial nitric-oxide synthase activity by increasing intracellular tetrahydrobiopterin. J Biol Chem 275:17399–17406
Ladurner A, Schmitt CA, Schachner D, Atanasov AG, Werner ER, Dirsch VM, Heiss EH (2012) Ascorbate stimulates endothelial nitric oxide synthase enzyme activity by rapid modulation of its phosphorylation status. Free Radic Biol Med 52:2082–2090
Baker TA, Milstien S, Katusic ZS (2001) Effect of vitamin C on the availability of tetrahydrobiopterin in human endothelial cells. J Cardiovasc Pharmacol 37:333–338
d’Uscio LV, Milstien S, Richardson D, Smith L, Katusic ZS (2003) Long-term vitamin C treatment increases vascular tetrahydrobiopterin levels and nitric oxide synthase activity. Circ Res 92:88–95
Nonami Y, Rao V, Shiono N, Ogoshi S (1998) Quenching the effects of L-arginine on free radical injury in cultured cardiomyocytes. Surg Today 28:379–384
Razavi HM, Hamilton JA, Feng Q (2005) Modulation of apoptosis by nitric oxide: implications in myocardial ischemia and heart failure. Pharmacol Ther 106:147–162
Barnabé N, Marusak RA, Hasinoff BB (2003) Prevention of doxorubicin-induced damage to rat heart myocytes by arginine analog nitric oxide synthase inhibitors and their enantiomers. Nitric Oxide 9:211–216
Kanno S, Lee PC, Zhang Y, Ho C, Griffith BP, Shears LL 2nd, Billiar TR (2000) Attenuation of of myocardial ischemia/reperfusion injury by superinduction of inducible nitric oxide synthase. Circulation 101:2742–2748
Wang P, Zweier JL (1996) Measurement of nitric oxide and peroxynitrite generation in the postischemic heart. J Biol Chem 271:29223–29230
Bloch W, Addicks K, Hescheler J, Fleischmann BK (2001) Nitric oxidesynthase expression and function in embryonic and adult cardiomyocytes. Microsc Res Tech 55:259–269
Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG (2003) Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 111:1201–1209
Otani H (2009) The role of nitric oxide in myocardial repair and remodeling. Antioxid Redox Signal 11:1913–1928
Visioli F, Smith A, Zhang W, Keaney JF Jr, Hagen T, Frei B (2002) Lipoic acid and vitamin C potentiate nitric oxide synthesis in human aortic endothelial cells independently of cellular glutathione status. Redox Rep 7:223–227
Okazaki T, Otani H, Shimazu T, Yoshioka K, Fujita M, Iwasaka T (2011) Ascorbic acid and N-acetyl cysteine prevent uncoupling of nitric oxide synthase and increase tolerance to ischemia/reperfusion injury in diabetic rat heart. Free Radic Res 45:1173–1183
Portugal CC, da Encarnação TG, Socodato R, Moreira SR, Brudzewsky D, Ambrósio AF, Paes-de-Carvalho R (2012) Nitric oxide modulates sodium vitamin C transporter 2 (SVCT-2) protein expression via protein kinase G (PKG) and nuclear factor-κB (NF-κB). J Biol Chem 287:3860–3872
Lowenstein CJ, Alley EW, Raval P, Snowman AM, Snyder SH, Russell SW, Murphy WJ (1993) Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon gamma and lipopolysaccharide. Proc Natl Acad Sci U S A 90:9730–9734
Xie Q-W, Kashiwabara Y, Nathan C (1994) Role of transcription factor NF-B/Rel in induction of nitric oxide synthase. J Biol Chem 269:4705–4708
Bolli R (2001) Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J Mol Cell Cardiol 33:1897–1918
Jones SP, Bolli R (2006) The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol 40:16–23
Zeglinski MR, Premecz S, Lerner J, Wtorek P, DaSilva M, Hsanally D, Chaudhary R, Sharma A, Thliveris J, Ravandi A, Singal PK, Jassal DS (2014) Congenital absence of nitric oxide synthase 3 potentiates cardiac dysfunction and reduces survival in doxorubicin- and trastuzumab-mediated cardiomyopathy. Can J Cardiol 30:359–367
Nossuli TO, Hayward R, Jensen D, Scalia R, Lefer AM (1998) Mechanisms of cardioprotection by peroxynitrite in myocardial ischemia and reperfusion injury. Am J Phys 275:H509–H519
Ellulu MS, Rahmat A, Patimah I, Khaza’ai H, Abed Y (2015) Effect of vitamin C on inflammation and metabolic markers in hypertensive and/or diabetic obese adults: a randomized controlled trial. Drug Des Devel Ther 9:3405–3412
El-Shitany NA, El-Desoky K (2016) Protective effects of carvedilol and vitamin C against azithromycin-induced cardiotoxicity in rats via decreasing ROS, IL1-beta, and TNF-alpha production and inhibiting NF-kappaB and caspase-3 expression. Oxidative Med Cell Longev 2016:1874762–1874713
Simone CB 2nd, Simone NL, Simone V, Simone CB (2007) Antioxidants and other nutrients do not interfere with chemotherapy or radiation therapy and can increase kill and increase survival, part 2. Altern Ther Health Med 13:40–47
Carr AC, Vissers MC, Cook JS (2014) The effect of intravenous vitamin C on cancer- and chemotherapy-related fatigue and quality of life. Front Oncol 4:283
Takahashi H, Mizuno H, Yanagisawa A (2012) High dose intravenous vitamin C improves quality of life in cancer patients. Personalized Med Univ 1:49–53
Yeom CH, Jung GC, Song KJ (2007) Changes of terminal cancer patients’ health-related quality of life after high dose vitamin C administration. J Korean Med Sci 22:7–11
Drisko JA, Chapman J, Hunter VJ (2003) The use of antioxidant therapies during chemotherapy. Gynecol Oncol 88:434–439
Ohno S, Ohno Y, Suzuki N, Soma G, Inoue M (2009) High-dose vitamin C (ascorbic acid) therapy in the treatment of patients with advanced cancer. Anticancer Res 29:809–815
Verrax J, Calderon PB (2009) Pharmacologic concentrations of ascorbate are achieved by parenteral administration and exhibit antitumoral effects. Free Radic Biol Med 47:32–40
Moss RW (2006) Should patients undergoing chemotherapy and radiotherapy be prescribed antioxidants? Integr Cancer Ther 5:63–82
Santos RV, Batista ML Jr, Caperuto EC, Costa Rosa LF (2007) Chronic supplementation of creatine and vitamins C and E increases survival and improves biochemical parameters after doxorubicin treatment in rats. Clin Exp Pharmacol Physiol 34:1294–1299
Yoshihara D, Fujiwara N, Suzuki K (2010) Antioxidants: benefits and risks for long-term health. Maturitas 67:103–107
Funding
The study was supported by operating grants from the Heart and Stroke Foundation of Canada, Molson’s Women Heart Health, and Canada Agriculture Partnership Programs. Dr. P.K. Singal is the holder of Dr. Naranjan S. Dhalla Chair in Cardiovascular Research supported by the St. Boniface Hospital Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
All authors have consented to participate.
Consent for publication
All authors have consented for publication.
Code availability
Not applicable.
Disclaimer
Submitted article represents an original work that is not being considered elsewhere for publication, in whole or in part. All previously published work cited in the manuscript has been fully acknowledged. The manuscript has been read and approved by all authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Figure 2 has unconverted data.
Rights and permissions
About this article
Cite this article
Malik, A., Bagchi, A.K., Vinayak, K. et al. Vitamin C: historical perspectives and heart failure. Heart Fail Rev 26, 699–709 (2021). https://doi.org/10.1007/s10741-020-10036-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-020-10036-y