Abstract
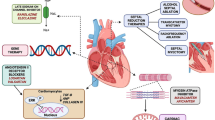
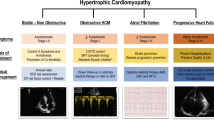
Hypertrophic cardiomyopathy is the most common inherited heart disease. Although it was first described over 50 years ago, there has been little in the way of novel disease-specific therapeutic development for these patients. Current treatment practice largely aims at symptomatic control using old drugs made for other diseases and does little to modify the disease course. Septal reduction by surgical myectomy or percutaneous alcohol septal ablation are well-established treatments for pharmacologic-refractory left ventricular outflow tract obstruction in hypertrophic cardiomyopathy patients. In recent years, there has been a relative surge in the development of innovative therapeutics, which aim to target the complex molecular pathophysiology and resulting hemodynamics that underlie hypertrophic cardiomyopathy. Herein, we review the new and emerging therapeutics for hypertrophic cardiomyopathy, which include pharmacologic attenuation of sarcomeric calcium sensitivity, allosteric inhibition of cardiac myosin, myocardial metabolic modulation, and renin-angiotensin-aldosterone system inhibition, as well as structural intervention by percutaneous mitral valve plication and endocardial radiofrequency ablation of septal hypertrophy. In conclusion, while further development of these therapeutic strategies is ongoing, they each mark a significant and promising advancement in treatment for hypertrophic cardiomyopathy patients.

Similar content being viewed by others
References
Maron BJ, Gardin JM, Flack JM et al (1995) Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Circulation 92:785–789. https://doi.org/10.1161/01.CIR.92.4.785
Maron BJ, Olivotto I, Spirito P et al (2000) Epidemiology of hypertrophic cardiomyopathy-related death: revisited in a large non-referral-based patient population. Circulation 102:858–864. https://doi.org/10.1161/01.CIR.102.8.858
Maron BJ, McKenna WJ, Danielson GK et al (2003) American College of Cardiology/European Society of Cardiology Clinical Expert Consensus Document on hypertrophic cardiomyopathy. J Am Coll Cardiol 42:1687–1713. https://doi.org/10.1016/S0735-1097(03)00941-0
Sen-Chowdhry S, Jacoby D, Moon JC, McKenna WJ (2016) Update on hypertrophic cardiomyopathy and a guide to the guidelines. Nat Rev Cardiol 13:651–675. https://doi.org/10.1038/nrcardio.2016.140
Maron MS, Olivotto I, Zenovich AG et al (2006) Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation 114:2232–2239. https://doi.org/10.1161/CIRCULATIONAHA.106.644682
Spoladore R, Maron MS, D’Amato R et al (2012) Pharmacological treatment options for hypertrophic cardiomyopathy: high time for evidence. Eur Heart J 33:1724–1733. https://doi.org/10.1093/eurheartj/ehs150
Ammirati E, Contri R, Coppini R et al (2016) Pharmacological treatment of hypertrophic cardiomyopathy: current practice and novel perspectives. Eur J Heart Fail 18:1106–1118. https://doi.org/10.1002/ejhf.541
Agarwal S, Tuzcu EM, Desai MY et al (2010) Updated meta-analysis of septal alcohol ablation versus myectomy for hypertrophic cardiomyopathy. J Am Coll Cardiol 55:823–834. https://doi.org/10.1016/j.jacc.2009.09.047
Valeti US, Nishimura RA, Holmes DR et al (2007) Comparison of surgical septal myectomy and alcohol septal ablation with cardiac magnetic resonance imaging in patients with hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol 49:350–357. https://doi.org/10.1016/j.jacc.2006.08.055
Liebregts M, Vriesendorp PA, Mahmoodi BK et al (2015) A systematic review and meta-analysis of long-term outcomes after septal reduction therapy in patients with hypertrophic cardiomyopathy. JACC Hear Fail 3:896–905. https://doi.org/10.1016/j.jchf.2015.06.011
Semsarian C, Ahmad I, Giewat M et al (2002) The L-type calcium channel inhibitor diltiazem prevents cardiomyopathy in a mouse model. J Clin Invest 109:1013–1020. https://doi.org/10.1172/JCI200214677
Ho CY, Lakdawala NK, Cirino AL et al (2015) Diltiazem treatment for pre-clinical hypertrophic cardiomyopathy sarcomere mutation carriers: a pilot randomized trial to modify disease expression. JACC Hear Fail 3:180–188. https://doi.org/10.1016/j.jchf.2014.08.003
Coppini R, Ferrantini C, Yao L et al (2013) Late sodium current inhibition reverses electromechanical dysfunction in human hypertrophic cardiomyopathy. Circulation 127:575–584. https://doi.org/10.1161/CIRCULATIONAHA.112.134932
Gentry JL, Mentz RJ, Hurdle M, Wang A (2016) Ranolazine for treatment of angina or dyspnea in hypertrophic cardiomyopathy patients (RHYME). J Am Coll Cardiol 68:1815–1817. https://doi.org/10.1016/j.jacc.2016.07.758
Bemporad D (2016) Ranolazine in patients with symptomatic hypertrophic cardiomyopathy: a pilot study assessing the effects on exercise capacity, diastolic function and symptomatic status. EU Clin Trials Regist. https://www.clinicaltrialsregister.eu/ctr-search/trial/2011-004507-20/DE. Accessed August 1, 2016
Gilead Sciences (2017) Effect of eleclazine (GS-6615) on exercise capacity in subjects with symptomatic hypertrophic cardiomyopathy (LIBERTY-HCM). US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT02291237. Accessed February 17, 2017
Gilead Sciences (2017) Effect of eleclazine on shortening of the QT interval, safety, and tolerability in adults with long QT syndrome type 3. US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT02300558?te. Accessed February 17, 2017
Nag S, Sommese RF, Ujfalusi Z et al (2015) Contractility parameters of human beta-cardiac myosin with the hypertrophic cardiomyopathy mutation R403Q show loss of motor function. Sci Adv 1:1–16. https://doi.org/10.1126/sciadv.1500511
Kamdar F, Klaassen Kamdar A, Koyano-Nakagawa N et al (2015) Cardiomyopathy in a dish: using human inducible pluripotent stem cells to model inherited cardiomyopathies. J Card Fail 21:761–770. https://doi.org/10.1016/j.cardfail.2015.04.010
Maslov MY, Chacko VP, Stuber M et al (2007) Altered high-energy phosphate metabolism predicts contractile dysfunction and subsequent ventricular remodeling in pressure-overload hypertrophy mice. Am J Physiol Heart Circ Physiol 292:H387–H391. https://doi.org/10.1152/ajpheart.00737.2006
Ferrantini C, Belus A, Piroddi N et al (2009) Mechanical and energetic consequences of HCM-causing mutations. J Cardiovasc Transl Res 2:441–451. https://doi.org/10.1007/s12265-009-9131-8
Wilder T, Ryba DM, Wieczorek DF et al (2015) N-acetylcysteine reverses diastolic dysfunction and hypertrophy in familial hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol. doi:https://doi.org/10.1152/ajpheart.00339.2015
Lee L, Campbell R, Scheuermann-Freestone M et al (2005) Metabolic modulation with perhexiline in chronic heart failure: a randomized, controlled trial of short-term use of a novel treatment. Circulation 112:3280–3288. https://doi.org/10.1161/CIRCULATIONAHA.105.551457
Horowitz JD, Sia STB, Macdonald PS et al (1986) Perhexiline maleate treatment for severe angina pectoris—correlations with pharmacokinetics. Int J Cardiol 13:219–229. https://doi.org/10.1016/0167-5273(86)90146-4
Olivotto I, Gistri R, Petrone P et al (2003) Maximum left ventricular thickness and risk of sudden death in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 41:315–321. https://doi.org/10.1016/S0735-1097(02)02713-4
Shimada YJ, Passeri JJ, Baggish AL et al (2013) Effects of losartan on left ventricular hypertrophy and fibrosis in patients with nonobstructive hypertrophic cardiomyopathy. JACC Hear Fail 1:480–487. https://doi.org/10.1016/j.jchf.2013.09.001
Axelsson A, Iversen K, Vejlstrup N et al (2015) Efficacy and safety of the angiotensin II receptor blocker losartan for hypertrophic cardiomyopathy: the INHERIT randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 3:123–131. https://doi.org/10.1016/S2213-8587(14)70241-4
Lim DS, Reynolds MR, Feldman T et al (2014) Improved functional status and quality of life in prohibitive surgical risk patients with degenerative mitral regurgitation after transcatheter mitral valve repair. J Am Coll Cardiol 64:182–192. https://doi.org/10.1016/j.jacc.2013.10.021
Maron BJ, Dearani JA, Ommen SR et al (2004) The case for surgery in obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 44:2044–2053. https://doi.org/10.1016/j.jacc.2004.04.063
Lawrenz T, Kuhn HJ (2004) Endocardial radiofrequency ablation of septal hypertrophy: a new catheter-based modality of gradient reduction in hypertrophic obstructive cardiomyopathy. Z Kardiol 93:493–499
Pohlmann L, Kröger I, Vignier N et al (2007) Cardiac myosin-binding protein C is required for complete relaxation in intact myocytes. Circ Res 101:928–938. https://doi.org/10.1161/CIRCRESAHA.107.158774
Iorga B, Blaudeck N, Solzin J et al (2008) Lys184 deletion in troponin I impairs relaxation kinetics and induces hypercontractility in murine cardiac myofibrils. Cardiovasc Res 77:676–686. https://doi.org/10.1093/cvr/cvm113
Huke S, Knollmann BC (2010) Increased myofilament Ca2+ sensitivity and arrhythmia susceptibility. J Mol Cell Cardiol 48:824–833. https://doi.org/10.1016/j.yjmcc.2010.01.011
Baudenbacher F, Schober T, Pinto JR et al (2008) Myofilament Ca sensitization causes susceptibility to cardiac arrhythmia in mice. J Clin Invest 118:3845–3903. https://doi.org/10.1172/JCI36642
Ho CY, Sweitzer NK, McDonough B et al (2002) Assessment of diastolic function with Doppler tissue imaging to predict genotype in preclinical hypertrophic cardiomyopathy. Circulation 105:2992–2997. https://doi.org/10.1161/01.CIR.0000019070.70491.6D
Nagueh SF, Bachinski LL, Meyer D et al (2001) Tissue Doppler imaging consistently detects myocardial abnormalities in patients with hypertrophic cardiomyopathy and provides a novel means for an early diagnosis before and independently of hypertrophy. Circulation 104:128–130. https://doi.org/10.1161/01.CIR.104.2.128
Michele D, Albayya FP, Metzger JM (1999) Direct, convergent hypersensitivity of calcium-activated force generation produced by hypertrophic cardiomyopathy mutant alpha-tropomyosins in adult cardiac myocytes. Nat Med 5:1413–1417. https://doi.org/10.1038/70990
Frey N, McKinsey TA, Olson EN (2000) Decoding calcium signals involved in cardiac growth and function. Nat Med 6:1221–1227. https://doi.org/10.1038/81321
Tardiff JC, Carrier L, Bers DM et al (2015) Targets for therapy in sarcomeric cardiomyopathies. Cardiovasc Res 105:457–470. https://doi.org/10.1093/cvr/cvv023
Robinson P, Griffiths PJ, Watkins H, Redwood CS (2007) Dilated and hypertrophic cardiomyopathy mutations in troponin and alpha-tropomyosin have opposing effects on the calcium affinity of cardiac thin filaments. Circ Res 101:1266–1273. https://doi.org/10.1161/CIRCRESAHA.107.156380
Fatkin D, Mcconnell BK, Mudd JO et al (2000) An abnormal Ca2+ response in mutant sarcomere protein-mediated familial hypertrophic cardiomyopathy. J Clin Invest 106:1351–1359. https://doi.org/10.1172/JCI11093
Lovelock JD, Monasky MM, Jeong EM et al (2012) Ranolazine improves cardiac diastolic dysfunction through modulation of myofilament calcium sensitivity. Circ Res 110:841–850. https://doi.org/10.1161/CIRCRESAHA.111.258251
Flenner F, Friedrich FW, Ungeheuer N et al (2016) Ranolazine antagonizes catecholamine-induced dysfunction in isolated cardiomyocytes, but lacks long-term therapeutic effects in vivo in a mouse model of hypertrophic cardiomyopathy. Cardiovasc Res 109:90–102. https://doi.org/10.1093/cvr/cvv247
Buvoli M, Hamady M, Leinwand LA, Knight R (2008) Bioinformatics assessment of β-myosin mutations reveals myosin’s high sensitivity to mutations. Trends Cardiovasc Med 18:141–149. https://doi.org/10.1016/j.tcm.2008.04.001
Kirschner SE, Becker E, Antognozzi M et al (2004) Hypertrophic cardiomyopathy-related-myosin mutations cause highly variable calcium sensitivity with functional imbalances among individual muscle cells. AJP Hear Circ Physiol 288:H1242–H1251. https://doi.org/10.1152/ajpheart.00686.2004
Spudich JA (2014) Hypertrophic and dilated cardiomyopathy: four decades of basic research on muscle lead to potential therapeutic approaches to these devastating genetic diseases. Biophys J 106:1236–1249. https://doi.org/10.1016/j.bpj.2014.02.011
Walsh R, Rutland C, Thomas R, Loughna S (2009) Cardiomyopathy: a systematic review of disease-causing mutations in myosin heavy chain 7 and their phenotypic manifestations. Cardiology 115:49–60. https://doi.org/10.1159/000252808
Green EM, Wakimoto H, Anderson RL et al (2016) A small-molecule inhibitor of sarcomere contractility suppresses hypertrophic cardiomyopathy in mice. Science 351(80):617–621
MyoKardia Inc (2015) MyoKardia provides update on two phase 1 trials of MYK-461 for the treatment of hypertrophic cardiomyopathy. In: http://investors.myokardia.com/phoenix.zhtml?c=254211&p=irol-newsArticle&ID=2097088
Witjas-Paalberends ER, Güclü A, Germans T et al (2014) Gene-specific increase in the energetic cost of contraction in hypertrophic cardiomyopathy caused by thick filament mutations. Cardiovasc Res 103:248–257. https://doi.org/10.1093/cvr/cvu127
Witjas-Paalberends ER, Ferrara C, Scellini B et al (2014) Faster cross-bridge detachment and increased tension cost in human hypertrophic cardiomyopathy with the R403Q MYH7 mutation. J Physiol 592:3257–3272. https://doi.org/10.1113/jphysiol.2014.274571
Ashrafian H, Redwood C, Blair E, Watkins H (2003) Hypertrophic cardiomyopathy: a paradigm for myocardial energy depletion. Trends Genet 19:263–268. https://doi.org/10.1016/S0168-9525(03)00081-7
Frey N, Olson EN (2003) Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol 65:45–79. https://doi.org/10.1146/annurev.physiol.65.092101.142243
Blair E, Redwood C, Ashrafian H et al (2001) Mutations in the γ 2 subunit of AMP-activated protein kinase cause familial hypertrophic cardiomyopathy: evidence for the central role of energy compromise in disease pathogenesis. Hum Mol Genet 10:1215–1220. https://doi.org/10.1093/hmg/10.11.1215
Crilley JG, Boehm EA, Blair E et al (2003) Hypertrophic cardiomyopathy due to sarcomeric gene mutations is characterized by impaired energy metabolism irrespective of the degree of hypertrophy. J Am Coll Cardiol 41:1776–1782. https://doi.org/10.1016/S0735-1097(02)03009-7
Unno K, Isobe S, Izawa H et al (2009) Relation of functional and morphological changes in mitochondria to myocardial contractile and relaxation reserves in asymptomatic to mildly symptomatic patients with hypertrophic cardiomyopathy. Eur Heart J 30:1853–1862. https://doi.org/10.1093/eurheartj/ehp184
Camici P, Chiriatti G, Lorenzoni R et al (1991) Coronary vasodilation is impaired in both hypertrophied and nonhypertrophied myocardium of patients with hypertrophic cardiomyopathy: a study with nitrogen-13 ammonia and positron emission tomography. J Am Coll Cardiol 17:879–886. https://doi.org/10.1016/0735-1097(91)90869-B
Cecchi F, Olivotto I, Gistri R et al (2003) Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N Engl J Med 349:1027–1035
Petersen SE, Jerosch-Herold M, Hudsmith LE et al (2007) Evidence for microvascular dysfunction in hypertrophic cardiomyopathy: new insights from multiparametric magnetic resonance imaging. Circulation 115:2418–2425. https://doi.org/10.1161/CIRCULATIONAHA.106.657023
Lombardi R, Rodriguez G, Chen SN et al (2009) Resolution of established cardiac hypertrophy and fibrosis and prevention of systolic dysfunction in a transgenic rabbit model of human cardiomyopathy through thiol-sensitive mechanisms. Circulation 119:1398–1407. https://doi.org/10.1161/CIRCULATIONAHA.108.790501.Resolution
Marian AJ (2015) Hypertrophic regression with N-Acetylcysteine in HCM (HALT). US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT01537926?. Accessed February 17, 2017
Lele SS, Thomson HL, Seo H et al (1995) Exercise capacity in hypertrophic cardiomyopathy. Circulation 92:2886–2894
Phan TT, Shivu GN, Abozguia K et al (2010) Impaired heart rate recovery and chronotropic incompetence in patients with heart failure with preserved ejection fraction. Circ Hear Fail 3:29–34. https://doi.org/10.1161/CIRCHEARTFAILURE.109.877720
Jeffrey FMH, Alvarez L, Diczku V et al (1995) Direct evidence that perhexiline modifies myocardial substrate utilization from fatty acids to lactate. J Cardiovasc Pharmacol 25:469–472
Abozguia K, Elliott P, McKenna W et al (2010) Metabolic modulator perhexiline corrects energy deficiency and improves exercise capacity in symptomatic hypertrophic cardiomyopathy. Circulation 122:1562–1569. https://doi.org/10.1161/CIRCULATIONAHA.109.934059
Cole PL, Beamer AD, Mcgowan N et al (1990) Efficacy and safet of perhexiline maleate refractory angina a double-blind placebo-controlled clinical trial of a novel. Circulation 81:1260–1270
Heart Metabolics Limited (2015) Efficacy, safety, and tolerability of perhexiline in subjects with hypertrophic cardiomyopathy and heart failure. US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT02431221. Accessed February 17, 2017
Varnava AM, Elliott PM, Sharma S et al (2000) Hypertrophic cardiomyopathy: the interrelation of disarray, fibrosis, and small vessel disease. Heart 84:476–482. https://doi.org/10.1136/heart.84.5.476
Marian AJ (2000) Pathogenesis of diverse clinical and pathological phenotypes in hypertrophic cardiomyopathy. Lancet 355:58–60. https://doi.org/10.1016/S0140-6736(99)06187-5
Spirito P, Chiarella F, Carratino L et al (1989) Clinical course and prognosis of hypertrophic cardiomyopathy in an outpatient population. N Engl J Med 320:749–755. https://doi.org/10.1056/NEJM198603273141302
Elliott PM, Gimeno Blanes JR, Mahon NG et al (2001) Relation between severity of left-ventricular hypertrophy and prognosis in patients with hypertrophic cardiomyopathy. Lancet 357:420–424. https://doi.org/10.1016/S0140-6736(00)04005-8
Maron BJ, Casey SA, Hauser RG, Aeppli DM (2003) Clinical course of hypertrophic cardiomyopathy with survival to advanced age. J Am Coll Cardiol 42:882–888. https://doi.org/10.1016/S0735-1097(03)00855-6
Teekakirikul P, Eminaga S, Toka O et al (2010) Cardiac fibrosis in mice with hypertrophic cardiomyopathy is mediated by non-myocyte proliferation and requires TGF-β. J Clin Invest 120:3520–3529. https://doi.org/10.1172/JCI42028DS1
Lim D, Lutucuta S, Bachireddy P et al (2001) Angiotensin II blockade reverses myocardial fibrosis in a transgenic mouse model of human hypertrophic cardiomyopathy. Circulation 103:789–792
Kawano H, Toda G, Nakamizo R et al (2005) Valsartan decreases type I collagen synthesis in patients with hypertrophic cardiomyopathy. Circ J 69:1244–1248
Araujo AQ, Arteaga E, Ianni BM et al (2005) Effect of losartan on left ventricular diastolic function in patients with nonobstructive hypertrophic cardiomyopathy. Am J Cardiol 96:1563–1567. https://doi.org/10.1016/j.amjcard.2005.07.065
Yamazaki T, Suzuki J-I, Shimamoto R et al (2007) A new therapeutic strategy for hypertrophic nonobstructive cardiomyopathy in humans. A randomized and prospective study with an angiotensin II receptor blocker. Int Heart J 48:715–724. https://doi.org/10.1536/ihj.48.715
Penicka M, Gregor P, Kerekes R et al (2009) The effects of candesartan on left ventricular hypertrophy and function in nonobstructive hypertrophic cardiomyopathy. J Mol Diagnostics 11:35–41. https://doi.org/10.2353/jmoldx.2009.080082
US National Library of Medicine (2016) clinicaltrials.gov https://clinicaltrials.gov/ct2/show/NCT01912534
Maron MS, Olivotto I, Betocchi S et al (2003) Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med 348:295–303. https://doi.org/10.1056/NEJMoa021332\r348/4/295
Wigle ED, Marquis Y, Auger P (1967) Muscular subaortic stenosis: initial left ventricular inflow tract pressure in the assessment of intraventricular pressures differences in man. Circulation 36:1100–1117
Ross J, Braunwald E, Gault JH et al (1966) The mechanism of the intraventricular pressure gradient in idiopathic hypertrophic subaortic stenosis. Circulation 34:558–578
Shah PM, Gramiak R, Kramer D (1969) Ultrasound localization of left ventricular outflow obstruction in hypertrophic obstructive cardiomyopathy. Circulation 40:3–11. https://doi.org/10.1161/01.CIR.40.1.3
Cape EG, Simons D, Jimoh A et al (1989) Chordal geometry determines the shape and extent of systolic anterior mitral motion: in vitro studies. J Am Coll Cardiol 13:1438–1448. https://doi.org/10.1016/0735-1097(89)90326-4
Sherrid MV, Balaram S, Kim B et al (2016) The mitral valve in obstructive hypertrophic cardiomyopathy: a test in context. J Am Coll Cardiol 67:1846–1858. https://doi.org/10.1016/j.jacc.2016.01.071
Spirito P, Maron BJ (1984) Patterns of systolic anterior motion of the mitral valve in hypertrophic cardiomyopathy: assessment by two-dimensional echocardiography. Am J Cardiol 54:1039–1046. https://doi.org/10.1016/S0002-9149(84)80141-1
Grigg LE, Wigle ED, Williams WG et al (1992) Transesophageal Doppler echocardiography in obstructive hypertrophic cardiomyopathy: clarification of pathophysiology and importance in intraoperative decision making. J Am Coll Cardiol 20:42–52. https://doi.org/10.1016/0735-1097(92)90135-A
Schwammenthal E, Nakatani S, He S et al (1998) Mechanism of mitral regurgitation in hypertrophic cardiomyopathy: mismatch of posterior to anterior leaflet length and mobility. Circulation 98:856–865
Yu EHC, Omran AS, Wigle ED et al (2000) Mitral regurgitation in hypertrophic obstructive cardiomyopathy: relationship to obstruction and relief with myectomy. J Am Coll Cardiol 36:2219–2225. https://doi.org/10.1016/S0735-1097(00)01019-6
Smedira NG, Lytle BW, Lever HM et al (2008) Current effectiveness and risks of isolated septal myectomy for hypertrophic obstructive cardiomyopathy. Ann Thorac Surg 85:127–133. https://doi.org/10.1016/j.athoracsur.2007.07.063
Balaram SK, Tyrie L, Sherrid MV et al (2008) Resection-plication-release for hypertrophic cardiomyopathy: clinical and echocardiographic follow-up. Ann Thorac Surg 86:1539–1545. https://doi.org/10.1016/j.athoracsur.2008.07.048
Balaram SK, Ross RE, Sherrid MV et al (2012) Role of mitral valve plication in the surgical management of hypertrophic cardiomyopathy. Ann Thorac Surg 94:1990–1998. https://doi.org/10.1016/j.athoracsur.2012.06.008
Shah AA, Glower DD, Gaca JG (2016) Trans-aortic Alfieri stitch at the time of septal myectomy for hypertrophic obstructive cardiomyopathy. J Card Surg 31:503–506. https://doi.org/10.1111/jocs.12804
Ferrazzi P, Spirito P, Iacovoni A et al (2015) Transaortic chordal cutting mitral valve repair for obstructive hypertrophic cardiomyopathy with mild septal hypertrophy. J Am Coll Cardiol 66:1687–1696. https://doi.org/10.1016/j.jacc.2015.07.069
Maron BJ, Dearani JA, Maron MS, et al (2017) Why we need more septal myectomy surgeons: an emerging recognition. J Thorac Cardiovasc Surg 1–6. doi:https://doi.org/10.1016/j.jtcvs.2016.12.038
Feldman T, Foster E, Glower DD et al (2011) Percutaneous repair or surgery for mitral regurgitation. N Engl J Med 364:1395–1406
Mauri L, Foster E, Glower DD et al (2013) 4-year results of a randomized controlled trial of percutaneous repair versus surgery for mitral regurgitation. J Am Coll Cardiol 62:317–328. https://doi.org/10.1016/j.jacc.2013.04.030
Sorajja P, Pedersen W, Bae R et al (2016) First experience with percutaneous mitral valve plication as primary therapy for symptomatic obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 67:1516. https://doi.org/10.1016/S0735-1097(16)31517-0
Jaber WA, Nishimura RA, Ommen SR (2007) Not all systolic velocities indicate obstruction in hypertrophic cardiomyopathy: a simultaneous Doppler catheterization study. J Am Soc Echocardiogr 20:5–7. https://doi.org/10.1016/j.echo.2007.01.015
Criley JM, Siegel RJ (1986) Obstruction is unimportant in the pathophysiology of hypertrophic cardiomyopathy. Postgrad Med J 62:515–529. https://doi.org/10.1136/pgmj.62.728.515
Murgo JP, Alter BR, Dorethy JF et al (1980) Dynamics of left ventricular ejection in obstructive and nonobstructive hypertrophic cardiomyopathy. J Clin Invest 66:1369–1382. https://doi.org/10.1172/JCI109990
Criley JM (1997) Unobstructed thinking (and terminology) is called for in the understanding and management of hypertrophic cardiomyopathy. J Am Coll Cardiol 29:741–743. https://doi.org/10.1016/S0735-1097(96)00590-6
Sahn DJ, Yoganathan AP, Editors G et al (1989) Pressure recovery distal to a stenosis: potential cause of a gradient “overestimation” by Doppler echocardiography. J Am Coll Cardiol 13:706–715
Braunwald E, Lambrew CT, Rockoff SD et al (1964) Idiopathic hypertrophic subaortic stenosis: I. A description of the disease based upon an analysis of 64 patients. Circulation 29:IV-3–IV-119. https://doi.org/10.1161/01.CIR.29.5S4.IV-3
Geske JB, Sorajja P, Ommen SR, Nishimura RA (2009) Left ventricular outflow tract gradient variability in hypertrophic cardiomyopathy. Clin Cardiol 32:397–402. https://doi.org/10.1002/clc.20594
Geske JB, Sorajja P, Ommen SR, Nishimura RA (2011) Variability of left ventricular outflow tract gradient during cardiac catheterization in patients with hypertrophic cardiomyopathy. JACC Cardiovasc Interv 4:704–709. https://doi.org/10.1016/j.jcin.2011.02.014
Veselka J, Jensen MK, Liebregts M et al (2016) Long-term clinical outcome after alcohol septal ablation for obstructive hypertrophic cardiomyopathy: results from the Euro-ASA registry. Eur Heart J 37:1517–1523. https://doi.org/10.1093/eurheartj/ehv693
Veselka J, Anavekar NS, Charron P (2017) Hypertrophic obstructive cardiomyopathy. Lancet 389:1253–1267. https://doi.org/10.1016/S0140-6736(16)31321-6
Lawrenz T, Borchert B, Leuner C et al (2011) Endocardial radiofrequency ablation for hypertrophic obstructive cardiomyopathy: acute results and 6 months’ follow-up in 19 patients. J Am Coll Cardiol 57:572–576. https://doi.org/10.1016/j.jacc.2010.07.055
Sreeram N, Emmel M, De Giovanni JV (2011) Percutaneous radiofrequency septal reduction for hypertrophic obstructive cardiomyopathy in children. J Am Coll Cardiol 58:2501–2510. https://doi.org/10.1016/j.jacc.2011.09.020
Cooper RM, Shahzad A, Hasleton J et al (2015) Radiofrequency ablation of the interventricular septum to treat outflow tract gradients in hypertrophic obstructive cardiomyopathy: a novel use of CARTOSound® technology to guide ablation. Eur Eur pacing, arrhythmias, Card Electrophysiol J Work groups Card pacing, arrhythmias, Card Cell Electrophysiol Eur Soc Cardiol 18:113–120. https://doi.org/10.1093/europace/euv302
Crossen K, Jones M, Erikson C (2016) Radiofrequency septal reduction in symptomatic hypertrophic obstructive cardiomyopathy. Hear Rhythm 13:1885–1890. https://doi.org/10.1016/j.hrthm.2016.04.018
Shelke AB, Menon R, Kapadiya A et al (2016) A novel approach in the use of radiofrequency catheter ablation of septal hypertrophy in hypertrophic obstructive cardiomyopathy. Indian Heart J 68:618–623. https://doi.org/10.1016/j.ihj.2016.02.007
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Philipson, D.J., DePasquale, E.C., Yang, E.H. et al. Emerging pharmacologic and structural therapies for hypertrophic cardiomyopathy. Heart Fail Rev 22, 879–888 (2017). https://doi.org/10.1007/s10741-017-9648-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-017-9648-x