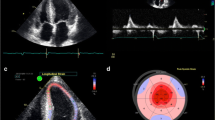
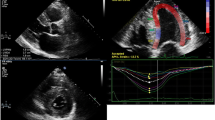
Abstract
The heart, like any organ in the body, is susceptible to amyloid deposition. Although more than 30 types of protein can cause amyloidosis, only two types commonly deposit in the ventricular myocardium: amyloid light chain and amyloid transthyretin. Amyloid cardiomyopathy is usually a major determinant of patient outcomes, and the diagnosis of heart involvement can be often relatively under-diagnosed, owing to nonspecific presenting symptoms and signs at a subclinical stage. The diagnosis of cardiac amyloidosis is usually performed by endomyocardial biopsy; however, the invasive nature and related high-risk complications restrict its wide use in clinical settings. Recently, with the advent of innovative techniques used for evaluating cardiac amyloidosis, noninvasive methods become increasingly important, especially in earlier diagnosis, distinguishing typing, risk prediction and response to treatment. Here, we will review recent developments in the noninvasive methods used in the assessment of cardiac amyloidosis, focused on the laboratory biomarkers and imaging modalities.






Similar content being viewed by others
References
Wechalekar AD, Gillmore JD, Hawkins PN (2015) Systemic amyloidosis. The Lancet. doi:10.1016/S0140-6736(15)01274-X
Sipe JD, Benson MD, Buxbaum JN et al (2014) Nomenclature 2014: amyloid fibril proteins and clinical classification of the amyloidosis. Amyloid 21:221–224
Yusuf SW, Solhpour A, Banchs J et al (2014) Cardiac amyloidosis. Expert Rev Cardiovasc Ther 12:265–277
Collins AB, Smith RN, Stone JR (2009) Classification of amyloid deposits in diagnostic cardiac specimens by immunofluorescence. Cardiovasc Pathol 18:205–216
Vrana JA, Gamez JD, Madden BJ et al (2009) Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood 114:4957–4959
Eulitz M, Weiss DT, Solomon A (1990) Immunoglobulin heavy-chain-associated amyloidosis. Proc Natl Acad Sci USA 87:6542–6546
Olson LJ, Gertz MA, Edwards WD et al (1987) Senile cardiac amyloidosis with myocardial dysfunction. Diagnosis by endomyocardial biopsy and immunohistochemistry. N Engl J Med 317:738–742
Millucci L, Ghezzi L, Paccagnini E et al (2014) Amyloidosis, inflammation, and oxidative stress in the heart of an alkaptonuric patient. Mediat Inflamm 2014:258471
Kawano M, Muramoto H, Yamada M et al (1998) Fatal cardiac β2-microglobulin amyloidosis in patients on long-term hemodialysis. Am J Kidney Dis 31:E4
Takayama F, Miyazaki S, Morita T et al (2001) Dialysis-related amyloidosis of the heart in long-term hemodialysis patients. Kidney Int Suppl 78:S172–S176
Valleix S, Gillmore JD, Bridoux F et al (2012) Hereditary systemic amyloidosis due to Asp76Asn variant β2-microglobulin. N Engl J Med 366:2276–2283
Hamidi AL, Liepnieks JJ, Hamidi AK et al (1999) Hereditary amyloid cardiomyopathy caused by a variant apolipoprotein A1. Am J Pathol 154:221–227
Yazaki M, Liepnieks JJ, Barats MS et al (2003) Hereditary systemic amyloidosis associated with a new apolipoprotein AII stop codon mutation Stop78Arg. Kidney Int 64:11–16
Bergstrom J, Murphy CL, Weiss DT et al (2004) Two different types of amyloid deposits—apolipoprotein A-IV and transthyretin—in a patient with systemic amyloidosis. Lab Investig 84:981–988
Maury CP, Baumann M (1990) Isolation and characterization of cardiac amyloid in familial amyloid polyneuropathy type IV (Finnish): relation of the amyloid protein to variant gelsolin. Biochim Biophys Acta 1096:84–86
Steiner I, Hajkova P (2006) Patterns of isolated atrial amyloid: a study of 100 hearts on autopsy. Cardiovasc Pathol 15:287–290
Maleszewski JJ (2015) Cardiac amyloidosis: pathology, nomenclature, and typing. Cardiovasc Pathol 24:343–350
Palladini G, Milani P, Merlini G (2015) Novel strategies for the diagnosis and treatment of cardiac amyloidosis. Expert Rev Cardiovasc Ther 13:1195–1211
Mohty D, Damy T, Cosnay P et al (2013) Cardiac amyloidosis: updates in diagnosis and management. Arch Cardiovasc Dis 106:528–540
Patel KS, Hawkins PN (2015) Cardiac amyloidosis: where are we today? J Intern Med 278:126–144
Kourelis TV, Gertz MA (2015) Improving strategies for the diagnosis of cardiac amyloidosis. Expert Rev Cardiovasc Ther 13:945–961
Kyle RA, Gertz MA (1995) Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Semin Hematol 32:45–59
Kyle RA, Linos A, Beard CM et al (1992) Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota, 1950 through 1989. Blood 79:1817–1822
Pinney JH, Smith CJ, Taube JB et al (2013) Systemic amyloidosis in England: an epidemiological study. Br J Haematol 161:525–532
Kaufman GP, Dispenzieri A, Gertz MA et al (2015) Kinetics of organ response and survival following normalization of the serum free light chain ratio in AL amyloidosis. Am J Hematol 90:181–186
Kourelis TV, Kumar SK, Go RS et al (2014) Immunoglobulin light chain amyloidosis is diagnosed late in patients with preexisting plasma cell dyscrasias. Am J Hematol 89:1051–1054
Kourelis TV, Kumar SK, Gertz MA et al (2013) Coexistent multiple myeloma or increased bone marrow plasma cells define equally high-risk populations in patients with immunoglobulin light chain amyloidosis. J Clin Oncol 31:4319–4324
McWilliams-Koeppen HP, Foster JS, Hackenbrack N et al (2015) Light chain amyloid fibrils cause metabolic dysfunction in human cardiomyocytes. PLoS ONE 10:e137716
Lavatelli F, Imperlini E, Orru S et al (2015) Novel mitochondrial protein interactors of immunoglobulin light chains causing heart amyloidosis. FASEB J 29:4614–4628
Guan J, Mishra S, Shi J et al (2013) Stanniocalcin1 is a key mediator of amyloidogenic light chain induced cardiotoxicity. Basic Res Cardiol 108:378
Wittich CM, Neben-Wittich MA, Mueller PS et al (2007) Deposition of amyloid proteins in the epicardial coronary arteries of 58 patients with primary systemic amyloidosis. Cardiovasc Pathol 16:75–78
Dorbala S, Vangala D, Bruyere JJ et al (2014) Coronary microvascular dysfunction is related to abnormalities in myocardial structure and function in cardiac amyloidosis. JACC Heart Fail 2:358–367
Sikkink LA, Ramirez-Alvarado M (2010) Cytotoxicity of amyloidogenic immunoglobulin light chains in cell culture. Cell Death Dis 1:e98
Shi J, Guan J, Jiang B et al (2010) Amyloidogenic light chains induce cardiomyocyte contractile dysfunction and apoptosis via a non-canonical p38 MAPK pathway. Proc Natl Acad Sci 107:4188–4193
Brenner DA, Jain M, Pimentel DR et al (2004) Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circ Res 94:1008–1010
Migrino RQ, Hari P, Gutterman DD et al (2010) Systemic and microvascular oxidative stress induced by light chain amyloidosis. Int J Cardiol 145:67–68
Diomede L, Rognoni P, Lavatelli F et al (2014) A Caenorhabditis elegans-based assay recognizes immunoglobulin light chains causing heart amyloidosis. Blood 123:3543–3552
Guan J, Mishra S, Qiu Y et al (2014) Lysosomal dysfunction and impaired autophagy underlie the pathogenesis of amyloidogenic light chain-mediated cardiotoxicity. EMBO Mol Med 6:1493–1507
Cooley CB, Ryno LM, Plate L et al (2014) Unfolded protein response activation reduces secretion and extracellular aggregation of amyloidogenic immunoglobulin light chain. Proc Natl Acad Sci USA 111:13046–13051
Ruberg FL, Berk JL (2012) Transthyretin (TTR) cardiac amyloidosis. Circulation 126:1286–1300
Ton VK, Mukherjee M, Judge DP (2014) Transthyretin cardiac amyloidosis: pathogenesis, treatments, and emerging role in heart failure with preserved ejection fraction. Clin Med Insights Cardiol 8:39–44
Dubrey S, Ackermann E, Gillmore J (2015) The transthyretin amyloidoses: advances in therapy. Postgrad Med J 91:439–448
Zeldenrust SR (2012) Genotype–phenotype correlation in FAP. Amyloid 19(Suppl 1):22–24
Castro-Rodrigues AF, Gales L, Saraiva MJ et al (2011) Structural insights into a zinc-dependent pathway leading to Leu55Pro transthyretin amyloid fibrils. Acta Crystallogr D Biol Crystallogr 67:1035–1044
Sousa MM, Fernandes R, Palha JA et al (2002) Evidence for early cytotoxic aggregates in transgenic mice for human transthyretin Leu55Pro. Am J Pathol 161:1935–1948
Jacobson DR, McFarlin DE, Kane I et al (1992) Transthyretin Pro55, a variant associated with early-onset, aggressive, diffuse amyloidosis with cardiac and neurologic involvement. Hum Genet 89:353–356
Yukio A, Yoshiki S, Konen O et al (2016) Effects of tafamidis treatment on transthyretin (TTR) stabilization, efficacy, and safety in Japanese patients with familial amyloid polyneuropathy (TTR-FAP) with Val30Met and non-Varl30Met: a phase III, open-label study. J Neurol Sci 362:266–271
Adams D, Coelho T, Obici L et al (2015) Rapid progression of familial amyloidotic polyneuropathy: a multinational natural history study. Neurology 85:675–682
Yamashita T, Hamidi AK, Yazaki M et al (2005) A prospective evaluation of the transthyretin Ile122 allele frequency in an African-American population. Amyloid 12:127–130
Buxbaum J, Alexander A, Koziol J et al (2010) Significance of the amyloidogenic transthyretin Val122 Ile allele in African Americans in the Arteriosclerosis Risk in Communities (ARIC) and Cardiovascular Health (CHS) Studies. Am Heart J 159:864–870
Ruberg FL, Maurer MS, Judge DP et al (2012) Prospective evaluation of the morbidity and mortality of wild-type and V122I mutant transthyretin amyloid cardiomyopathy: the Transthyretin Amyloidosis Cardiac Study (TRACS). Am Heart J 164:222–228
Quarta CC, Buxbaum JN, Shah AM et al (2015) The amyloidogenic V122I transthyretin variant in elderly black Americans. N Engl J Med 372:21–29
Soares ML, Coelho T, Sousa A et al (2004) Haplotypes and DNA sequence variation within and surrounding the transthyretin gene: genotype-phenotype correlations in familial amyloid polyneuropathy (V30M) in Portugal and Sweden. Eur J Hum Genet 12:225–237
Plante-Bordeneuve V, Kerschen P (2013) Transthyretin familial amyloid polyneuropathy. Handb Clin Neurol 115:643–658
Rapezzi C, Quarta CC, Riva L et al (2010) Transthyretin-related amyloidoses and the heart: a clinical overview. Nat Rev Cardiol 7:398–408
Greene MJ, Sam F, Soo HP et al (2011) Evidence for a functional role of the molecular chaperone clusterin in amyloidotic cardiomyopathy. Am J Pathol 178:61–68
Sorgjerd K, Ghafouri B, Jonsson BH et al (2006) Retention of misfolded mutant transthyretin by the chaperone BiP/GRP78 mitigates amyloidogenesis. J Mol Biol 356:469–482
Sousa MM, Yan SD, Stern D et al (2000) Interaction of the receptor for advanced glycation end products (RAGE) with transthyretin triggers nuclear transcription factor kB (NF-kB) activation. Lab Investig 80:1101–1110
Yan SF, Ramasamy R, Schmidt AM (2010) The RAGE axis: a fundamental mechanism signaling danger to the vulnerable vasculature. Circ Res 106:842–853
Biolo A, Ramamurthy S, Connors LH et al (2008) Matrix metalloproteinases and their tissue inhibitors in cardiac amyloidosis: relationship to structural, functional myocardial changes and to light chain amyloid deposition. Circ Heart Fail 1:249–257
Pomerance A (1965) Senile cardiac amyloidosis. Br Heart J 27:711–718
Mohammed SF, Mirzoyev SA, Edwards WD et al (2014) Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart Fail 2:113–122
Goncalves I, Alves CH, Quintela T et al (2008) Transthyretin is up-regulated by sex hormones in mice liver. Mol Cell Biochem 317:137–142
Bochtler T, Hegenbart U, Heiss C et al (2008) Evaluation of the serum-free light chain test in untreated patients with AL amyloidosis. Haematologica 93:459–462
Palladini G, Dispenzieri A, Gertz MA et al (2012) New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol 30:4541–4549
Gertz MA (2014) Immunoglobulin light chain amyloidosis: 2014 update on diagnosis, prognosis, and treatment. Am J Hematol 89:1132–1140
Usuku H, Obayashi K, Shono M et al (2013) Usefulness of plasma B-type natriuretic peptide as a prognostic marker of cardiac function in senile systemic amyloidosis and in familial amyloidotic polyneuropathy. Amyloid 20:251–255
Lehrke S, Steen H, Kristen AV et al (2009) Serum levels of NT-proBNP as surrogate for cardiac amyloid burden: new evidence from gadolinium-enhanced cardiac magnetic resonance imaging in patients with amyloidosis. Amyloid 16:187–195
Palladini G, Campana C, Klersy C et al (2003) Serum N-terminal pro-brain natriuretic peptide is a sensitive marker of myocardial dysfunction in AL amyloidosis. Circulation 107:2440–2445
Apridonidze T, Steingart RM, Comenzo RL et al (2012) Clinical and echocardiographic correlates of elevated troponin in amyloid light-chain cardiac amyloidosis. Am J Cardiol 110:1180–1184
Kumar S, Dispenzieri A, Lacy MQ et al (2012) Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol 30:989–995
Cheng Z, Zhu K, Tian Z et al (2013) The findings of electrocardiography in patients with cardiac amyloidosis. Ann Noninvasive Electrocardiol 18:157–162
Rahman JE, Helou EF, Gelzer-Bell R et al (2004) Noninvasive diagnosis of biopsy-proven cardiac amyloidosis. J Am Coll Cardiol 43:410–415
Cyrille NB, Goldsmith J, Alvarez J et al (2014) Prevalence and prognostic significance of low QRS voltage among the three main types of cardiac amyloidosis. Am J Cardiol 114:1089–1093
Mussinelli R, Salinaro F, Alogna A et al (2013) Diagnostic and prognostic value of low QRS voltages in cardiac AL amyloidosis. Ann Noninvasive Electrocardiol 18:271–280
Zhao L, Li J, Tian Z et al (2015) Clinical correlates and prognostic values of pseudoinfarction in cardiac light-chain amyloidosis. J Cardiol. doi:10.1016/j.jjcc.2015.11.004
Neben-Wittich MA, Wittich CM, Mueller PS et al (2005) Obstructive intramural coronary amyloidosis and myocardial ischemia are common in primary amyloidosis. Am J Med 118:1281–1287
Boldrini M, Salinaro F, Mussinelli R et al (2013) Prevalence and prognostic value of conduction disturbances at the time of diagnosis of cardiac AL amyloidosis. Ann Noninvasive Electrocardiol 18:327–335
Rapezzi C, Merlini G, Quarta CC et al (2009) Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation 120:1203–1212
Perlini S, Salinaro F, Cappelli F et al (2013) Prognostic value of fragmented QRS in cardiac AL amyloidosis. Int J Cardiol 167:2156–2161
Ariyarajah V, Steiner I, Hajkova P et al (2009) The association of atrial tachyarrhythmias with isolated atrial amyloid disease: preliminary observations in autopsied heart specimens. Cardiology 113:132–137
Longhi S, Quarta CC, Milandri A et al (2015) Atrial fibrillation in amyloidotic cardiomyopathy: prevalence, incidence, risk factors and prognostic role. Amyloid 22:147–155
Reyners AK, Hazenberg BP, Reitsma WD et al (2002) Heart rate variability as a predictor of mortality in patients with AA and AL amyloidosis. Eur Heart J 23:157–161
Barbhaiya CR, Kumar S, Baldinger SH et al (2016) Electrophysiologic assessment of conduction abnormalities and atrial arrhythmias associated with amyloid cardiomyopathy. Heart Rhythm 13:383–390
Chew C, Ziady GM, Raphael MJ et al (1975) The functional defect in amyloid heart disease. The “stiff heart” syndrome. Am J Cardiol 36:438–444
St JSM, Reichek N, Kastor JA et al (1982) Computerized M-mode echocardiographic analysis of left ventricular dysfunction in cardiac amyloid. Circulation 66:790–799
Koyama J, Ikeda S, Ikeda U (2015) Echocardiographic assessment of the cardiac amyloidoses. Circ J 79:721–734
Di Bella G, Pizzino F, Minutoli F et al (2014) The mosaic of the cardiac amyloidosis diagnosis: role of imaging in subtypes and stages of the disease. Eur Heart J Cardiovasc Imaging 15:1307–1315
Banypersad SM, Moon JC, Whelan C et al (2012) Updates in cardiac amyloidosis: a review. J Am Heart Assoc 1:e364
Gertz MA, Comenzo R, Falk RH et al (2005) Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th international symposium on amyloid and amyloidosis, tours, france, 18–22 April 2004. Am J Hematol 79:319–328
Carroll JD, Gaasch WH, McAdam KP (1982) Amyloid cardiomyopathy: characterization by a distinctive voltage/mass relation. Am J Cardiol 49:9–13
Falk RH, Plehn JF, Deering T et al (1987) Sensitivity and specificity of the echocardiographic features of cardiac amyloidosis. Am J Cardiol 59:418–422
Zhao L, Tian Z, Fang Q (2016) Risk factors and prognostic role of left atrial enlargement in patients with cardiac light-chain amyloidosis. Am J Med Sci 351:271–278
Lee GY, Kim K, Choi J et al (2014) Cardiac amyloidosis without increased left ventricular wall thickness. Mayo Clin Proc 89:781–789
Suresh R, Grogan M, Maleszewski JJ et al (2014) Advanced cardiac amyloidosis associated with normal interventricular septal thickness: an uncommon presentation of infiltrative cardiomyopathy. J Am Soc Echocardiogr 27:440–447
Tei C, Dujardin KS, Hodge DO et al (1996) Doppler index combining systolic and diastolic myocardial performance: clinical value in cardiac amyloidosis. J Am Coll Cardiol 28:658–664
Porciani MC, Lilli A, Perfetto F et al (2009) Tissue Doppler and strain imaging: a new tool for early detection of cardiac amyloidosis. Amyloid 16:63–70
Ha J, Ommen SR, Tajik AJ et al (2004) Differentiation of constrictive pericarditis from restrictive cardiomyopathy using mitral annular velocity by tissue Doppler echocardiography. Am J Cardiol 94:316–319
Pokharel P, Fujikura K, Bella JN (2015) Clinical applications and prognostic implications of strain and strain rate imaging. Expert Rev Cardiovasc Ther 13:853–866
Koyama J, Ray-Sequin PA, Falk RH (2003) Longitudinal myocardial function assessed by tissue velocity, strain, and strain rate tissue Doppler echocardiography in patients with AL (primary) cardiac amyloidosis. Circulation 107:2446–2452
Koyama J, Falk RH (2010) Prognostic significance of strain doppler imaging in light-chain amyloidosis. JACC Cardiovasc Imaging 3:333–342
Bellavia D, Pellikka PA, Dispenzieri A et al (2012) Comparison of right ventricular longitudinal strain imaging, tricuspid annular plane systolic excursion, and cardiac biomarkers for early diagnosis of cardiac involvement and risk stratification in primary systematic (AL) amyloidosis: a 5-year cohort study. Eur Heart J Cardiovasc Imaging 13:680–689
Cacciapuoti F (2015) The role of echocardiography in the non-invasive diagnosis of cardiac amyloidosis. J Echocardiogr 13:84–89
Huang H, Jing X, Hu Z et al (2015) Early impairment of cardiac function and asynchronization of systemic amyloidosis with preserved ejection fraction using two-dimensional speckle tracking echocardiography. Echocardiography 32:1832–1840
Quarta CC, Solomon SD, Uraizee I et al (2014) Left ventricular structure and function in transthyretin-related versus light-chain cardiac amyloidosis. Circulation 129:1840–1849
Urbano-Moral JA, Gangadharamurthy D, Comenzo RL et al (2015) Three-dimensional speckle tracking echocardiography in light chain cardiac amyloidosis: examination of left and right ventricular myocardial mechanics parameters. Revista Española de Cardiología 68:657–664 (English Edition)
Sun JP, Stewart WJ, Yang XS et al (2009) Differentiation of hypertrophic cardiomyopathy and cardiac amyloidosis from other causes of ventricular wall thickening by two-dimensional strain imaging echocardiography. Am J Cardiol 103:411–415
Phelan D, Collier P, Thavendiranathan P et al (2012) Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart 98:1442–1448
Senapati A, Sperry BW, Grodin JL et al (2016) Prognostic implication of relative regional strain ratio in cardiac amyloidosis. Heart 102:748–754
Buss SJ, Emami M, Mereles D et al (2012) Longitudinal left ventricular function for prediction of survival in systemic light-chain amyloidosis: incremental value compared with clinical and biochemical markers. J Am Coll Cardiol 60:1067–1076
Liu D, Hu K, Niemann M et al (2013) Impact of regional left ventricular function on outcome for patients with AL amyloidosis. PLoS ONE 8:e56923
Fontana M, Chung R, Hawkins PN et al (2015) Cardiovascular magnetic resonance for amyloidosis. Heart Fail Rev 20:133–144
Li R, Yang Z, Wen L et al (2016) Regional myocardial microvascular dysfunction in cardiac amyloid light-chain amyloidosis: assessment with 3T cardiovascular magnetic resonance. J Cardiovasc Magn Reson 18:16
Syed IS, Glockner JF, Feng D et al (2010) Role of cardiac magnetic resonance imaging in the detection of cardiac amyloidosis. JACC Cardiovasc Imaging 3:155–164
Vogelsberg H, Mahrholdt H, Deluigi CC et al (2008) Cardiovascular magnetic resonance in clinically suspected cardiac amyloidosis. J Am Coll Cardiol 51:1022–1030
Ruberg FL, Appelbaum E, Davidoff R et al (2009) Diagnostic and prognostic utility of cardiovascular magnetic resonance imaging in light-chain cardiac amyloidosis. Am J Cardiol 103:544–549
Aquaro GD, Pugliese NR, Perfetto F et al (2014) Myocardial signal intensity decay after gadolinium injection: a fast and effective method for the diagnosis of cardiac amyloidosis. Int J Cardiovasc Imaging 30:1105–1115
Bhuva AN, Treibel TA, Fontana M et al (2014) T1 mapping: non-invasive evaluation of myocardial tissue composition by cardiovascular magnetic resonance. Expert Rev Cardiovasc Ther 12:1455–1464
Karamitsos TD, Piechnik SK, Banypersad SM et al (2013) Noncontrast T1 mapping for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging 6:488–497
Fontana M, Banypersad SM, Treibel TA et al (2014) Native T1 mapping in transthyretin amyloidosis. JACC Cardiovasc Imaging 7:157–165
Hosch W, Bock M, Libicher M et al (2007) MR-relaxometry of myocardial tissue: significant elevation of T1 and T2 relaxation times in cardiac amyloidosis. Investig Radiol 42:636–642
Banypersad SM, Sado DM, Flett AS et al (2013) Quantification of myocardial extracellular volume fraction in systemic AL amyloidosis: an equilibrium contrast cardiovascular magnetic resonance study. Circ Cardiovasc Imaging 6:34–39
Mongeon FP, Jerosch-Herold M, Coelho-Filho OR et al (2012) Quantification of extracellular matrix expansion by CMR in infiltrative heart disease. JACC Cardiovasc Imaging 5:897–907
Fontana M, Banypersad SM, Treibel TA et al (2015) Differential myocyte responses in patients with cardiac transthyretin amyloidosis and light-chain amyloidosis: a cardiac MR imaging study. Radiology 277:388–397
Boynton SJ, Geske JB, Dispenzieri A et al (2016) LGE provides incremental prognostic information over serum biomarkers in AL cardiac amyloidosis. JACC Cardiovasc Imaging. doi:10.1016/j.jcmg.2015.10.027
Fontana M, Pica S, Reant P et al (2015) Prognostic value of late gadolinium enhancement cardiovascular magnetic resonance in cardiac amyloidosis. Circulation 132:1570–1579
Banypersad SM, Fontana M, Maestrini V et al (2015) T1 mapping and survival in systemic light-chain amyloidosis. Eur Heart J 36:244–251
Kammerlander AA, Marzluf BA, Zotter-Tufaro C et al (2016) T1 mapping by CMR imaging: from histological validation to clinical implication. JACC Cardiovasc Imaging 9:14–23
Kristen AV, Aus Dem Siepen F, Scherer K et al (2014) Comparison of different types of cardiac amyloidosis by cardiac magnetic resonance imaging. Amyloid 22:132–141
Bokhari S, Shahzad R, Castaño A et al (2014) Nuclear imaging modalities for cardiac amyloidosis. J Nucl Cardiol 21:175–184
Kristen AV, Haufe S, Schonland SO et al (2013) Skeletal scintigraphy indicates disease severity of cardiac involvement in patients with senile systemic amyloidosis. Int J Cardiol 164:179–184
Perugini E, Guidalotti PL, Salvi F et al (2005) Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol 46:1076–1084
Rapezzi C, Quarta CC, Guidalotti PL et al (2011) Role of 99mTc-DPD scintigraphy in diagnosis and prognosis of hereditary transthyretin-related cardiac amyloidosis. JACC Cardiovasc Imaging 4:659–670
Hutt DF, Quigley AM, Page J et al (2014) Utility and limitations of 3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in systemic amyloidosis. Eur Heart J Cardiovasc Imaging 15:1289–1298
Yamamoto Y, Onoguchi M, Haramoto M et al (2012) Novel method for quantitative evaluation of cardiac amyloidosis using 201TlCl and 99mTc-PYP SPECT. Ann Nucl Med 26:634–643
Bokhari S, Castano A, Pozniakoff T et al (2013) 99mTc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging 6:195–201
Castaño A, DeLuca A, Weinberg R et al (2015) Serial scanning with technetium pyrophosphate (99mTc-PYP) in advanced ATTR cardiac amyloidosis. J Nucl Cardiol. doi:10.1007/s12350-015-0261-x
Harb SC, Haq M, Flood K et al (2016) National patterns in imaging utilization for diagnosis of cardiac amyloidosis: a focus on Tc99m-pyrophosphate scintigraphy. J Nucl Cardiol. doi:10.1007/s12350-016-0478-3
Nakata T, Shimamoto K, Yonekura S et al (1995) Cardiac sympathetic denervation in transthyretin-related familial amyloidotic polyneuropathy: detection with iodine-123-MIBG. J Nucl Med 36:1040–1042
Noordzij W, Glaudemans AWJM et al (2012) 123I-Labelled metaiodobenzylguanidine for the evaluation of cardiac sympathetic denervation in early stage amyloidosis. Eur J Nucl Med Mol I 39:1609–1617
Tanaka M, Hongo M, Kinoshita O et al (1997) Iodine-123 metaiodobenzylguanidine scintigraphic assessment of myocardial sympathetic innervation in patients with familial amyloid polyneuropathy. J Am Coll Cardiol 29:168–174
Coutinho MCA, Cortez-Dias N, Cantinho G et al (2013) Reduced myocardial 123-Iodine metaiodobenzylguanidine uptake: a prognostic marker in familial amyloid polyneuropathy. Circ Cardiovasc Imaging 6:627–636
Takahashi R, Ono K, Shibata S et al (2014) Efficacy of diflunisal on autonomic dysfunction of late-onset familial amyloid polyneuropathy (TTR Val30Met) in a Japanese endemic area. J Neurol Sci 345:231–235
Hongo M, Urushibata K, Kai R et al (2002) Iodine-123 metaiodobenzylguanidine scintigraphic analysis of myocardial sympathetic innervation in patients with AL (primary) amyloidosis. Am Heart J 144:122–129
Aprile C, Marinone G, Saponaro R et al (1995) Cardiac and pleuropulmonary AL amyloid imaging with technetium-99m labelled aprotinin. Eur J Nucl Med 22:1393–1401
Han S, Chong V, Murray T et al (2007) Preliminary experience of 99mTc‐aprotinin scintigraphy in amyloidosis. Eur J Haematol 79:494–500
Rowczenio D, Tennent GA, Gilbertson J et al (2014) Clinical characteristics and SAP scintigraphic findings in 10 patients with AGel amyloidosis. Amyloid 21:276–281
Richards DB, Cookson LM, Berges AC et al (2015) Therapeutic clearance of amyloid by antibodies to serum amyloid P component. New Engl J Med 373:1106–1114
Antoni G, Lubberink M, Estrada S et al (2013) In vivo visualization of amyloid deposits in the heart with 11C-PIB and PET. J Nucl Med 54:213–220
Lee SP, Lee ES, Choi H et al (2015) 11C-Pittsburgh B PET imaging in cardiac amyloidosis. JACC Cardiovasc Imaging 8:50–59
Dorbala S, Vangala D, Semer J et al (2014) Imaging cardiac amyloidosis: a pilot study using 18F-florbetapir positron emission tomography. Eur J Nucl Med Mol I 41:1652–1662
Law WP, Wang WY, Moore PT et al (2016) Cardiac amyloid imaging with 18F-florbetaben positron emission tomography: a pilot study. J Nucl Med. doi:10.2967/jnumed.115.169870
Van Der Gucht A, Galat A, Rosso J et al (2015) [18F]-NaF PET/CT imaging in cardiac amyloidosis. J Nucl Cardiol. doi:10.1007/s12350-015-0287-0
Gagliardi C, Tabacchi E, Bonfiglioli R et al (2016) Does the etiology of cardiac amyloidosis determine the myocardial uptake of [18F]-NaF PET/CT? J Nucl Cardiol. doi:10.1007/s12350-016-0457-8
Acknowledgments
We would like to thank Dr William Y.S. Wang, Dr. Arnold C.T. Ng, Dr. Peter T. Moore (Cardiology Department, Princess Alexandra Hospital, Australia) and Dr. W. Phillip Law (Medical Imaging Department, Princess Alexandra Hospital, Australia) for providing us with the images included in this review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhao, L., Fang, Q. Recent advances in the noninvasive strategies of cardiac amyloidosis. Heart Fail Rev 21, 703–721 (2016). https://doi.org/10.1007/s10741-016-9580-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-016-9580-5