Abstract
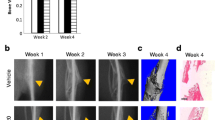
This paper explores the potential therapeutic role of the naturally occurring sugar heparan sulfate (HS) for the augmentation of bone repair. Scaffolds comprising fibrin glue loaded with 5 μg of embryonically derived HS were assessed, firstly as a release-reservoir, and secondly as a scaffold to stimulate bone regeneration in a critical size rat cranial defect. We show HS-loaded scaffolds have a uniform distribution of HS, which was readily released with a typical burst phase, quickly followed by a prolonged delivery lasting several days. Importantly, the released HS contributed to improved wound healing over a 3-month period as determined by microcomputed tomography (μCT) scanning, histology, histomorphometry, and PCR for osteogenic markers. In all cases, only minimal healing was observed after 1 and 3 months in the absence of HS. In contrast, marked healing was observed by 3 months following HS treatment, with nearly full closure of the defect site. PCR analysis showed significant increases in the gene expression of the osteogenic markers Runx2, alkaline phosphatase, and osteopontin in the heparin sulfate group compared with controls. These results further emphasize the important role HS plays in augmenting wound healing, and its successful delivery in a hydrogel provides a novel alternative to autologous bone graft and growth factor-based therapies.






Similar content being viewed by others
References
Babensee JE, McIntyre LV, Mikos AG (2000) Growth factor delivery for tissue engineering. Pharm Res 17:497–504
Blanquaert F, Saffar JL, Colombier ML, Carpentier G, Barritault D, Caruelle JP (1995) Heparan-like molecules induce the repair of skull defects. Bone 17:499–506
Choi KY, Kim HJ, Lee MH, Kwon TG, Nah HD, Furuichi T, Komori T, Nam SH, Kim YJ, Kim HJ, Ryoo HM (2005) Runx2 regulates FGF2-induced Bmp2 expression during cranial bone development. Dev Dyn 233(1):115–121
Cool SM, Nurcombe V (2005) The osteoblast-heparan sulfate axis: control of the bone cell lineage. Int J BiochemCell Biol 37(9):1739–1745
Coombe DR, Kett WC (2005) Heparan sulfate-protein interactions: therapeutic potential through structure-function insights. Cell Mol Life Sci 62:410–424
DeBlois C, Cote MF, Doillon CJ (1994) Heparin-fibroblast growth factor fibrin complex: in vitro and in vivo applications to collagen based materials. Biomaterials 15(9):665–672
Depprich R, Handschel J, Sebald W, Kubler NR, Wurzler KK (2005) Comparison of the osteogenic activity of bone morphogenetic protein (BMP) mutants. Mund Kiefer Gesichtschir 9:363–368
Ducy P, Starbuck M, Priemel M, Shen J, Pinero G, Geoffroy V, Amling M, Karsenty G (1999) A Cbfa1-dependent genetic pathway controls bone formation beyond embryonic development. Genes Dev 13(8):1025–1036
Fakhry A (2005) Effects of FGF-2/-9 in calvarial bone cell cultures: differentiation stage-dependent mitogenic effect, inverse regulation of BMP-2 and noggin, and enhancement of osteogenic potential. Bone 36:254–266
Guido D, Bernfield M (1998) The emerging roles of cell surface heparan sulfate proteoglycans. Matrix Biol 17(7):461–463
Irie A, Habuchi H, Kimata K, Sanai Y (2003) Heparan sulfate is required for bone morphogenetic protein-7 signaling. Biochem Biophys Res Commun 308:858–865
Jackson RA, McDonald MM, Nurcombe V, Little DG, Cool SM (2006a) The use of heparan sulfate to augment fracture repair in a rat fracture model. J Orthop Res 24:636–644
Jackson RA, Nurcombe V, Cool SM (2006b) Coordinated fibroblast growth factor and heparan sulfate regulation of Osteogenesis. Gene 379:79–91
Kasai S, Kunimoto T, Nitta K (1983) Cross linking of fibrin by activated factor XIII stimulates attachment, morphological changes and proliferation of fibroblasts. Biomed Res 4:155–160
Keller J, Anderasses TT, Joyce F (1985) Fixation of osteochondral fractures: fibrin sealant tested in dogs. Acta Orthop Scand 56:323–326
Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T (1997) Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89(5):755–764
Lafont J, Blanquaert F, Colombier ML, Barritault D, Carueelle JP, Saffar JL (2004) Kinetic study of early regenerative effects of RGTA11, a heparan sulfate mimetic, in rat craniotomy defects. Calcif Tissue Int 75:517–525
Le Nihouannen D, Le Guehennen L, Rouillon T, Pilet P, Bilban M, Layrolle P, Daculsi G (2006) Micro-architecture of calcium phosphate granules and fibrin glue composites for bone tissue engineering. Biomaterials 27:2716–2722
Luong-Van E, Grøndahl L, Nurcombe V, Cool SM (2007) In vitro biocompatibility and bioactivity of microencapsulated heparan sulfate. Biomaterials 28:2127–2136
Lyon M, Gallagher JT (1998) Bio-specific sequences and domains in heparan sulfate and the regulation of cell growth and adhesion. Matrix Biol 17(7):485–493
Marktl W, Rudas B (1974) The effect of factor XIII on wound granulation in the rat. Thromb Diath Haemorrh 32:578–581
Mundlos S, Otto F, Mundlos C, Mulliken JB, Aylsworth AS, Albright S, Lindhout D, Cole WG, Henn W, Knoll JH, Owen MJ, Mertelsmann R, Zabel BU, Olsen BR (1997) Mutations involving the transcription factor CBFA1 cause cleidocranial dysplasia. Cell 89(5):773–779
Ng KW, Spiecher T, Dombrowski C, Helledie T, Haupt LM, Nurcombe V, Cool SM (2007) Osteogenic differentiation of murine embryonic stem cells is mediated by fibroblast growth factor receptors. Stem Cells Dev 16:305–318
Ornitz DM, Marie PJ (2002) FGF signaling pathways in endochondral and intra-membranous bone development and human genetic disease. Genes Dev 16:1446–1465
Osmond RI, Kett WC, Skett SE, Coombe DR (2002) Protein-heparin interactions measured by BIAcore 2000 are affected by the method of heparin immobilization. Anal Biochem 310:199–207
Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, Selby PB, Owen MJ (1997) Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 89(5):765–771
Pandit AS, Wilson DJ, Feldman DS (2000) Fibrin scaffold as an effective vehicle for the delivery of acidic growth factor (FGF-1). J Biomater Appl 14(3):229–242
Pratap J, Galindo M, Zaidi SK, Vradii D, Bhat BM, Robinson JA, Choi JY, Komori T, Stein JL, Lian JB, Stein GS, van Wijnen AJ (2003) Cell growth regulatory cell regulatory role of Runx2 during proliferative expansion of preosteoblasts. Cancer Res 63(17):5357–5362
Rai B, Teoh SH, Hutmacher DW, Cao T, Ho KH (2005) Novel PCL-based honeycomb scaffolds as drug delivery systems for rhBMP-2. Biomaterials 26:3739–3748
Ruppert R, Hoffmann E, Sebald W (1996). Human bone morphogenetic protein 2 contains a heparin-binding site which modifies its biological activity. Eur J Biochem 237:295–302
Saksela O, Rifkin D (1989). Release of basic fibroblast growth factor-heparan sulfate complexes from endothelial cells by plasminogen activator-mediated proteolytic activity. Fibrinolysis 3:36
Shao XX, Hutmacher DW, Ho ST, Goh JC, Lee EH (2006) Evaluation of a hybrid scaffold/cell construct in repair of high-load-bearing osteochondral defects in rabbits. Biomaterials 27(7):1071–1080
Shireman PK, Greisler HP (1998) Fibrin Sealant in vascular surgery: a review. J Long Term Eff Med Implants 8:117–132
Takada T, Katagiri T, Ifuku M, Morimura N, Kobayashi M, Hasegawa K, Ogamo A, Kamijo R (2003) Sulfated polysaccharides enhance the biological activities of bone morphogenetic proteins. J Biol Chem 278:43229–43235
Turnbull J, Powell A, Guimond S (2001). Heparan sulfate: decoding a dynamic multifunctional cell regulator. Trends Cell Biol 11(2):75–82
Vasita R, Katti DS (2006) Growth factor delivery systems for tissue engineering: a materials perspective. Expert Rev Med Devices 3(1):29–47
Zhang X, Sobue T, Hurley MM (2002) FGF-2 increases colony formation, PTH receptor, and IGF-1 mRNA in mouse marrow stromal cells. Biochem Biophys Res Commun 290:526–531
Acknowledgments
The authors would like to thank Amber Annettte Sawyer for training with Bioquant image analysis software.
Author information
Authors and Affiliations
Corresponding author
Additional information
Maria Ann Woodruff and Subha Narayan Rath contributed equally to this work.
Rights and permissions
About this article
Cite this article
Woodruff, M.A., Rath, S.N., Susanto, E. et al. Sustained release and osteogenic potential of heparan sulfate-doped fibrin glue scaffolds within a rat cranial model. J Mol Hist 38, 425–433 (2007). https://doi.org/10.1007/s10735-007-9137-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-007-9137-y