Abstract
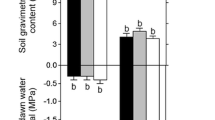
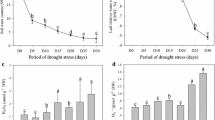
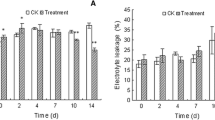
Grafting rootstocks are widely used to enhance plants resistance to various biologic and abiotic stresses. We determined how the rootstock genotype might influence plant responses to drought, using 2-year-old ‘Gale Gala’ apple trees grafted onto Malus sieversii and M. hupehensis. Under water stress, trees with the former as their rootstock had smaller reductions in rates of relative growth and photosynthesis, total biomass, leaf area, levels of leaf chlorophyll, and relative water content compared with those grafted onto the latter. They also had greater maximum photochemical efficiency and water-use efficiency. On the other hand, trees growing on M. sieversii rootstock had less production of superoxide radicals and hydrogen peroxide in both leaves and roots than those growing on M. hupehensis in response to drought stress. Furthermore, under drought conditions, leaves and roots from trees grafted onto M. sieversii had greater synthesis of ascorbic acid and glutathione, as well as higher activities of superoxide dismutase, catalase, ascorbate peroxidase, monodehydroascorbate reductase, dehydroascorbate reductase, and glutathione reductase. These results suggest that the choice of grafting rootstock can enhance drought resistance by improving the antioxidant system in a plant. Here, ‘Gale Gala’ trees grafted onto M. sieversii were more drought-resistant than those on M. hupehensis rootstock.





Similar content being viewed by others
Abbreviations
- ABA:
-
Abscisic acid
- APX:
-
Ascorbate peroxidase
- AsA:
-
Reduced ascorbate
- CAT:
-
Catalase
- DHA:
-
Dehydroascorbate
- DHAR:
-
Dehydroascorbate reductase
- GR:
-
Glutathione reductase
- GSH:
-
Reduced glutathione
- GSSG:
-
Oxidized glutathione
- H2O2 :
-
Hydrogen peroxide
- MDHAR:
-
Monodehydroascorbate reductase
- O ·−2 :
-
Superoxide anion
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
References
Aloni B, Cohen R, Karni L, Aktas H, Edelstein M (2010) Hormonal signaling in rootstock–scion interactions. Scientia Hortic 127:119–126
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Asada K (1999) The water–water cycle in chloroplasts: scavenging of active oxygen and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Bai TH, Li CY, Ma FW, Feng FJ, Shu HR (2010) Responses of growth and antioxidant system to root-zone hypoxia stress in two Malus species. Plant Soil 327:95–105
Bian SM, Jiang YW (2009) Reactive oxygen species, antioxidant enzyme activities and gene expression patterns in leaves and roots of Kentucky bluegrass in response to drought stress and recovery. Scientia Hortic 120:264–270
Bowler C, van Montagu M, Inze D (1992) Superoxide dismutase and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol 43:83–116
Chance M, Maehly AC (1955) Assay of catalases and peroxidases. Meth Enzymol 2:764–817
Cohen S, Naor A (2002) The effect of three rootstocks on water use, canopy conductance and hydraulic parameters of apple trees and predicting canopy from hydraulic conductance. Plant Cell Environ 25:17–28
Davies WJ, Zhang JH (1991) Root signals and the regulation of growth and development of plants in drying soil. Annu Rev Plant Physiol Plant Mol Biol 42:55–76
Dhindsa RS, Plumb-Dhindsa P, Throne TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Foyer CH (1993) Ascorbic acid. In: Alscher RG, Hess JL (eds) Antioxidants in higher plants. CRC Press, Boca Raton, pp 31–58
Foyer CH, Halliwell B (1976) Presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Foyer CH, Noctor G (2005) Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant, Cell Environ 28:1056–1071
Garg N, Manchanda G (2009) ROS generation in plants: boon or bane? Plant Biosyst 143:81–96
Gijón MC, Gimenez C, Perez-López D, Guerrero J, Couceiro JF, Moriana A (2010) Rootstock influences the response of pistachio (Pistacia vera L. cv. Kerman) to water stress and rehydration. Scientia Hortic 125:666–671
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Jiang MY, Zhang JH (2002) Role of abscisic acid in water stress-induced antioxidant defence in leaves of maize seedlings. Free Radical Res 36:1001–1015
Jubany-Marí T, Munné-Bosch S, Alegre L (2010) Redox regulation of water stress responses in field-grown plants. Role of hydrogen peroxide and ascorbate. Plant Physiol Biochem 48:351–358
Kampfenkel K, van Montagu M, Inze D (1995) Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal Biochem 225:165–167
Lee JM, Kubota C, Tsao SJ, Bie Z, Hoyos Echevarria P, Morra L, Oda M (2010) Current status of vegetable grafting: diffusion, grafting techniques, automation. Scientia Hortic 127:93–105
Li Y, Zhao HX, Duan BL, Korpelainen H, Li CY (2011) Effect of drought and ABA on growth, photosynthesis and antioxidant system of Cotinus coggygria seedling under two different light conditions. Environ Exp Bot 71:107–113
Lichtenthaler HK, Rinderle U (1988) The role of chlorophyll fluorescence in the detection of stress conditions in plants. Crit Rev Anal Chem 19:529–581
Ma XW, Ma FW, Mi YF, Ma YH, Shu HR (2008) Morphological and physiological responses of two contrasting Malus species to exogenous abscisic acid application. Plant Growth Regul 56:77–87
Ma XW, Ma FW, Li CY, Mi YF, Bai TH, Shu HR (2010) Biomass accumulation, allocation, and water-use efficiency in 10 Malus rootstocks under two watering regimes. Agroforest Syst 80:283–294
Martínez-Ballesta MC, Alcaraz-López C, Muries B, Mota-Cadenas C, Carvajal M (2010) Physiological aspects of rootstock–scion interactions. Scientia Hortic 127:112–118
Menconi M, Sgherri CLM, Pinzino C, Navari-Izzo F (1995) Activated oxygen production and detoxification in wheat plants subjected to a water deficit programme. J Exp Bot 46:1123–1130
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Møller IM, Jensen PE, Hansson A (2007) Oxidative modifications to cellular components in plants. Annu Rev Plant Biol 58:459–481
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Navrot N, Rouhier N, Gelhaye E, Jaquot JP (2007) Reactive oxygen species generation and antioxidant systems in plant mitochondria. Physiol Plant 129:185–195
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Pascual I, Azcona I, Morales F, Aguirreolea J, Sánchez-Díaz M (2010) Photosynthetic response of pepper plants to wilt induced by Verticillium dahliae and soil water deficit. J Plant Physiol 167:701–708
Rampino P, Pataleo S, Gerardi C, Mita G, Perrotta C (2006) Drought stress response in wheat: physiological and molecular analysis of resistant and sensitive genotypes. Plant Cell Environ 29:2143–2152
Rodríguez-Gamir J, Intrigliolo DS, Primo-Millo E, Forner-Giner MA (2010) Relationships between xylem anatomy, root hydraulic conductivity, leaf/root ratio and transpiration in citrus trees on different rootstocks. Physiol Plant 139:159–169
Sánchez-Rodríguez E, Rubio-Wilhelmi MM, Cervilla LM, Blasco B, Rios JJ, Rosales MA, Romero L, Ruiz JM (2010) Genotypic differences in some physiological parameters symptomatic for oxidative stress under moderate drought in tomato plants. Plant Sci 178:30–40
Schachtman DP, Goodger JQ (2008) Chemical root to shoot signaling under drought. Trends Plant Sci 13:281–287
Schwarz D, Rouphael Y, Colla G, Venema JH (2010) Grafting as a tool to improve tolerance of vegetables to abiotic stresses: thermal stress, water stress and organic pollutants. Scientia Hortic 127:162–171
Sgherri CLM, Navari-Izzo F (1995) Sunflower seedling subjected to increasing water deficit stress: oxidative stress and defence mechanisms. Physiol Plant 93:25–30
Smirnoff N (1993) The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol 125:27–58
Smirnoff N (1996) The function and metabolism of ascorbic acid in plants. Ann Bot 78:661–669
Sofo A, Tuzio AC, Dichio B, Xiloyannis C (2005) Influence of water deficit and rewatering on the components of the ascorbate-glutathione cycle in four interspecific Prunus hybrids. Plant Sci 169:403–412
Solari LI, Johnson S, DeJong TM (2006) Relationship of water status to vegetative growth and leaf gas exchange of peach (Prunus persica) trees on different rootstocks. Tree Physiol 26:1333–1341
Souza RP, Machado EC, Silva JAB, Lagoa A, Silveira JAG (2004) Photosynthetic gas exchange, chlorophyll fluorescence and some associated metabolic changes in cowpea (Vigna unguiculata) during water stress and recovery. Environ Exp Bot 51:45–56
Weibel A, Johnson RS, DeJong TM (2003) Comparative vegetative growth responses of two peach cultivars grown on size-controlling versus standard rootstocks. J Am Soc Hortic Sci 128:463–471
Wu FZ, Bao WK, Li FL, Wu N (2008) Effects of drought stress and N supply on the growth, biomass partitioning and water-use efficiency of Sophora davidii seedlings. Environ Exp Bot 63:248–255
Zhang J, Tardieu F (1996) Relative contribution of apices and mature tissues to ABA synthesis in drought maize root systems. Plant Cell Physiol 37:598–605
Zhang JX, Kirkham MB (1996) Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytol 132:361–373
Zhang ZK, Liu SQ, Hao SQ, Liu SH (2010) Grafting increases the copper tolerance of cucumber seedlings by improvement of polyamine contents and enhancement of antioxidant enzymes activity. Agric Sci China 9:985–994
Zhou ZQ (1999) The apple genetic resources in China: the wild species and their distributions, informative characteristics and utilization. Genet Resour Crop Evol 46:599–609
Acknowledgements
This work was supported by the earmarked fund for China Agriculture Research System. The authors are grateful to Priscilla Licht for help in revising our English composition and to Mr. Xuanchang Fu for management of the potted apple plants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, B., Li, M., Cheng, L. et al. Influence of rootstock on antioxidant system in leaves and roots of young apple trees in response to drought stress. Plant Growth Regul 67, 247–256 (2012). https://doi.org/10.1007/s10725-012-9683-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-012-9683-5