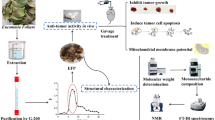
Abstract
A novel cold-water-soluble polysaccharide (BEP), with a molecular weight of 6.0 × 106 Da, was isolated from Boletus edulis. BEP consists of galactose, glucose, xylose, mannose, glucuronic, and galacturonic acid in a ratio of 0.34:0.28:0.28:2.57:1.00:0.44. The IR results showed that BEP was an acid polysaccharide, containing α-type and β-type glucoside bonds. MTT assay showed BEP could inhibit cell proliferation significantly. Morphological observation demonstrated that BEP-treated MDA-MB-231 and Ca761 cells exhibited typical apoptotic morphological features. Flow cytometry analysis revealed that BEP caused mitochondrial membrane potential collapse. Annexin V-FITC/PI staining indicated that BEP induced apoptosis of MDA-MB-231 and Ca761 cells through cell block in S phase and G0/G1 phase, respectively. Western blot results showed that BEP could increase the Bax/Bcl-2 ratios, promote the release of cytochrome C, and activate the expression of caspase-3 and caspase-9 in MDA-MB-231 and Ca761 cells. In conclusion, our results demonstrated that BEP could inhibit the proliferation of breast cancer cells and induce apoptosis through mitochondrial pathways.






Similar content being viewed by others
References
Monnot, G.C., Romero, P.: Rationale for immunological approaches to breast cancer therapy. Breast. 37, 187–195 (2018). https://doi.org/10.1016/j.breast.2017.06.009
Wang, X., Yang, Y., An, Y., Fang, G.: The mechanism of anticancer action and potential clinical use of kaempferol in the treatment of breast cancer. Biomed. Pharmacother. 117, 109086 (2019). https://doi.org/10.1016/j.biopha.2019.109086
Goldhirsch, A., Winer, E.P., Coates, A.S., Gelber, R.D., Piccart-Gebhart, M., Thurlimann, B., Senn, H.J.: Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 24(9), 2206–2223 (2013). https://doi.org/10.1093/annonc/mdt303
Bernier, J., Poortmans, P.M.: Surgery and radiation therapy of triple-negative breast cancers: from biology to clinics. Breast. 28, 148–155 (2016). https://doi.org/10.1016/j.breast.2016.05.014
Bauer, K.R., Brown, M., Cress, R.D., Parise, C.A., Caggiano, V.: Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer registry. Cancer. 109(9), 1721–1728 (2007). https://doi.org/10.1002/cncr.22618
Meshkat, B., Prichard, R.S., Al-Hilli, Z., Bass, G.A., Quinn, C., O’Doherty, A., Rothwell, J., Geraghty, J., Evoy, D., McDermott, E.W.: A comparison of clinical-pathological characteristics between symptomatic and interval breast cancer. Breast. 24(3), 278–282 (2015). https://doi.org/10.1016/j.breast.2015.02.032
Moran, M.S., Yang, Q., Harris, L.N., Jones, B., Tuck, D.P., Haffty, B.G.: Long-term outcomes and clinicopathologic differences of African-American versus white patients treated with breast conservation therapy for early-stage breast cancer. Cancer. 113(9), 2565–2574 (2008). https://doi.org/10.1002/cncr.23881
Tajbakhsh, A., Rivandi, M., Abedini, S., Pasdar, A., Sahebkar, A.: Regulators and mechanisms of anoikis in triple-negative breast cancer (TNBC): a review. Crit. Rev. Oncol. Hematol. 140, 17–27 (2019). https://doi.org/10.1016/j.critrevonc.2019.05.009
Shamsaei, S., Getso, M., Ahmadikia, K., Yarahmadi, M., Farahani, H.E., Aslani, R., Mohammadzade, A.S., Raissi, V., Soleimani, A., Arghavan, B., Karami, S., Mohseni, M., Mohseni, M.S., Raiesi, O.: Recent findings on the role of fungal products in the treatment of cancer. Clin. Transl. Oncol. (2020). https://doi.org/10.1007/s12094-020-02428-1
MacFarlane, M., Williams, A.C.: Apoptosis and disease: a life or death decision. EMBO Rep. 5(7), 674–678 (2004). https://doi.org/10.1038/sj.embor.7400191
Tang, X., Tang, J., Liu, X., Zeng, L., Cheng, C., Luo, Y., Li, L., Qin, S.L., Sang, Y., Deng, L.M., Lv, X.B.: Downregulation of miR-129-2 by promoter hypermethylation regulates breast cancer cell proliferation and apoptosis. Oncol. Rep. 35(5), 2963–2969 (2016). https://doi.org/10.3892/or.2016.4647
von Schwarzenberg, K., Vollmar, A.M.: Targeting apoptosis pathways by natural compounds in cancer: marine compounds as lead structures and chemical tools for cancer therapy. Cancer Lett. 332(2), 295–303 (2013). https://doi.org/10.1016/j.canlet.2010.07.004
Nicholson, D.W., Thornberry, N.A.: Caspases: killer proteases. Trends Biochem. Sci. 22(8), 299–306 (1997). https://doi.org/10.1016/S0968-0004(97)01085-2
Duprez, L., Wirawan, E., Vanden Berghe, T., Vandenabeele, P.: Major cell death pathways at a glance. Microbes Infect. 11(13), 1050–1062 (2009). https://doi.org/10.1016/j.micinf.2009.08.013
Levine, B., Sinha, S.C., Kroemer, G.J.A.: Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy. 4(5), 600–606 (2008). https://doi.org/10.4161/auto.6260
Yan, X., Wang, L., Yang, X., Qiu, Y., Tian, X., Lv, Y., Tian, F., Song, G., Wang, T.: Fluoride induces apoptosis in H9c2 cardiomyocytes via the mitochondrial pathway. Chemosphere. 182, 159–165 (2017). https://doi.org/10.1016/j.chemosphere.2017.05.002
Cui, L., Bu, W., Song, J., Feng, L., Xu, T., Liu, D., Ding, W., Wang, J., Li, C., Ma, B., Luo, Y., Jiang, Z., Wang, C., Chen, J., Hou, J., Yan, H., Yang, L., Jia, X.: Apoptosis induction by alantolactone in breast cancer MDA-MB-231 cells through reactive oxygen species-mediated mitochondrion-dependent pathway. Arch Pharm Res. 41(3), 299–313 (2018). https://doi.org/10.1007/s12272-017-0990-2
Manzi, P., Gambelli, L., Marconi, S., Vivanti, V., Pizzoferrato, L.: Nutrients in edible mushrooms: an inter-species comparative study. Food Chem. 65(4), 477–482 (1999). https://doi.org/10.1016/S0308-8146(98)00212-X
Kalač, P.: Chemical composition and nutritional value of European species of wild grow-ing mushrooms: a review. Food Chem. 113(1), 9–16 (2009). https://doi.org/10.1016/j.foodchem.2008.07.077
Ruthes, A.C., Smiderle, F.R., Iacomini, M.: d-Glucans from edible mushrooms: A review on the extraction, purification and chemical characterization approaches. Carbohydr. Polym. 117, 753–761 (2015). https://doi.org/10.1016/j.carbpol.2014.10.051
Zhang, H., Pu, D., Sun, B., Ren, F., Zhang, Y., Chen, H.: Characterization and comparison of key aroma compounds in raw and dry porcini mushroom (Boletus edulis) by aroma extract dilution analysis, quantitation and aroma recombination experiments. Food Chem. 258, 260–268 (2018). https://doi.org/10.1016/j.foodchem.2018.03.056
Dentinger, B.T., Ammirati, J.F., Both, E.E., Desjardin, D.E., Halling, R.E., Henkel, T.W., Moreau, P.A., Nagasawa, E., Soytong, K., Taylor, A.F., Watling, R., Moncalvo, J.M., McLaughlin, D.J.: Molecular phylogenetics of porcini mushrooms (Boletus section Boletus). Mol. Phylogenet. Evol. 57(3), 1276–1292 (2010). https://doi.org/10.1016/j.ympev.2010.10.004
Chen, W., Wang, W.P., Zhang, H.S., Huang, Q.: Optimization of ultrasonic-assisted extraction of water-soluble polysaccharides from Boletus edulis mycelia using response surface methodology. Carbohydr. Polym. 87(1), 614–619 (2012). https://doi.org/10.1016/j.carbpol.2011.08.029
Zhang, A.Q., Liu, Y., Xiao, N.N., Zhang, Y., Sun, P.L.: Structural investigation of a novel heteropolysaccharide from the fruiting bodies of Boletus edulis. Food Chem. 146, 334–338 (2014). https://doi.org/10.1016/j.foodchem.2013.09.073
Zhang, A., Xiao, N., He, P., Sun, P.: Chemical analysis and antioxidant activity in vitro of polysaccharides extracted from Boletus edulis. Int. J. Biol. Macromol. 49(5), 1092–1095 (2011). https://doi.org/10.1016/j.ijbiomac.2011.09.005
Lemieszek, M.K., Cardoso, C., Ferreira Milheiro Nunes, F.H., Ramos Novo Amorim de Barros, A.I., Marques, G., Pozarowski, P., Rzeski, W.: Boletus edulis biologically active biopolymers induce cell cycle arrest in human colon adenocarcinoma cells. Food Funct. 4(4), 575–585 (2013). https://doi.org/10.1039/c2fo30324h
Wang, D., Sun, S.Q., Wu, W.Z., Yang, S.L., Tan, J.M.: Characterization of a water-soluble polysaccharide from Boletus edulis and its antitumor and immunomodulatory activities on renal cancer in mice. Carbohydr. Polym. 105, 127–134 (2014). https://doi.org/10.1016/j.carbpol.2013.12.085
Zhang, L., Zhang, Q., Zheng, Y., He, Z., Guan, P., He, X., Hui, L., Dai, Y.: Study of Schiff base formation between dialdehyde cellulose and proteins, and its application for the deproteinization of crude polysaccharide extracts. Ind. Crop. Prod. 112, 532–540 (2018). https://doi.org/10.1016/j.indcrop.2017.12.056
Dubois, M., Gilles, H.A., Hamilton, J.K., Rebers, P.A., Smith, F.: Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 22–25 (1956). https://doi.org/10.1021/ac60111a017
Barbosa, H., Slater, N.K., Marcos, J.C.: Protein quantification in the presence of poly(ethylene glycol) and dextran using the Bradford method. Anal. Biochem. 395(1), 108–110 (2009). https://doi.org/10.1016/j.ab.2009.07.045
Bitter, T., Muir, H.M.: A modified uronic acid carbazole reaction. Anal. Biochem. 4(4), 330–334 (1962). https://doi.org/10.1016/0003-2697(62)90095-7
Yu, J., Ji, H.Y., Liu, A.J.: Alcohol-soluble polysaccharide from Astragalus membranaceus: Preparation, characteristics and antitumor activity. Int. J. Biol. Macromol. 118(Pt B), 2057–2064 (2018). https://doi.org/10.1016/j.ijbiomac.2018.07.073
Yu, J., Ji, H., Dong, X., Feng, Y., Liu, A.: Apoptosis of human gastric carcinoma MGC-803 cells induced by a novel Astragalus membranaceus polysaccharide via intrinsic mitochondrial pathways. Int. J. Biol. Macromol. 126, 811–819 (2019). https://doi.org/10.1016/j.ijbiomac.2018.12.268
Yu, J., Ji, H., Yang, Z., Liu, A.: Relationship between structural properties and antitumor activity of Astragalus polysaccharides extracted with different temperatures. Int. J. Biol. Macromol. 124, 469–477 (2019). https://doi.org/10.1016/j.ijbiomac.2018.11.156
Wang, Y., Li, Y., Liu, Y., Chen, X., Wei, X.: Extraction, characterization and antioxidant activities of se-enriched tea polysaccharides. Int. J. Biol. Macromol. 77, 76–84 (2015). https://doi.org/10.1016/j.ijbiomac.2015.02.052
Zhao, Y., Liu, Y., Wang, W., Wu, D., Shi, J., Liu, A.: Apoptosis and autophagy induction of Seleno-β-lactoglobulin (Se-β-Lg) on hepatocellular carcinoma cells lines. J. Funct. Foods 49, 412–423 (2018). https://doi.org/10.1016/j.jff.2018.09.011
Zhang, L., Li, X., Deng, H., Jing, Y., Fu, Q.: Enhanced thermal conductivity and electrical insulation properties of polymer composites via constructing Pglass/CNTs confined hybrid fillers. Compos. A: Appl. Sci. Manuf. 115, 1–7 (2018). https://doi.org/10.1016/j.compositesa.2018.09.009
Kong, L., Yu, L., Feng, T., Yin, X., Liu, T., Dong, L.: Physicochemical characterization of the polysaccharide from Bletilla striata: effect of drying method. Carbohydr. Polym. 125, 1–8 (2015). https://doi.org/10.1016/j.carbpol.2015.02.042
Jose, G.M., Raghavankutty, M., Kurup, G.M.: Sulfated polysaccharides from Padina tetrastromatica induce apoptosis in HeLa cells through ROS triggered mitochondrial pathway. Process Biochem. 68, 197–204 (2018). https://doi.org/10.1016/j.procbio.2018.02.014
Chen, D., Sun, S., Cai, D., Kong, G.: Induction of mitochondrial-dependent apoptosis in T24 cells by a selenium (Se)-containing polysaccharide from Ginkgo biloba L. leaves. Int. J. Biol. Macromol. 101, 126–130 (2017). https://doi.org/10.1016/j.ijbiomac.2017.03.033
Cui, H., Wang, C., Wang, Y., Li, Z., Zhang, Y., Chen, M., Li, F.: Pleurotus nebrodensis polysaccharide induces apoptosis in human non-small cell lung cancer A549 cells. Carbohydr. Polym. 104, 246–252 (2014). https://doi.org/10.1016/j.carbpol.2014.01.001
Azzopardi, M., Farrugia, G., Balzan, R.: Cell-cycle involvement in autophagy and apoptosis in yeast. Mech. Ageing Dev. 161(Pt B), 211–224 (2017). https://doi.org/10.1016/j.mad.2016.07.006
Wang, Y., Huo, T., Feng, C., Zeng, Y., Yang, J., Zhang, X., Dong, F., Deng, J.: Chrysotile asbestos induces apoptosis via activation of the p53-regulated mitochondrial pathway mediated by ROS in A549 cells. Appl. Clay Sci. 182, 105245 (2019). https://doi.org/10.1016/j.clay.2019.105245
Chen, G., Zhang, P., Huang, T., Yu, W., Lin, J., Li, P., Chen, K.: Polysaccharides from Rhizopus nigricans mycelia induced apoptosis and G2/M arrest in BGC-823 cells. Carbohydr. Polym. 97(2), 800–808 (2013). https://doi.org/10.1016/j.carbpol.2013.05.068
Wang, A., Si, Z., Xue, P., Li, X., Liu, J.: Tacrolimus protects hippocampal neurons of rats with status epilepticus through suppressing oxidative stress and inhibiting mitochondrial pathway of apoptosis. Brain Res. 1715, 176–181 (2019). https://doi.org/10.1016/j.brainres.2019.02.031
Gross, A.: BCL-2 family proteins as regulators of mitochondria metabolism. Biochim. Biophys. Acta 1857(8), 1243–1246 (2016). https://doi.org/10.1016/j.bbabio.2016.01.017
Du, L., Fei, Z., Song, S., Wei, N.: Antitumor activity of Lobaplatin against esophageal squamous cell carcinoma through caspase-dependent apoptosis and increasing the Bax/Bcl-2 ratio. Biomed. Pharmacother. 95, 447–452 (2017). https://doi.org/10.1016/j.biopha.2017.08.119
Vucicevic, K., Jakovljevic, V., Colovic, N., Tosic, N., Kostic, T., Glumac, I., Pavlovic, S., Karan-Djurasevic, T., Colovic, M.: Association of Bax expression and Bcl2/Bax ratio with clinical and molecular prognostic markers in chronic lymphocytic leukemia. J. Med. Biochem. 35(2), 150–157 (2016). https://doi.org/10.1515/jomb-2015-0017
Yao, W., Lin, Z., Shi, P., Chen, B., Wang, G., Huang, J., Sui, Y., Liu, Q., Li, S., Lin, X., Yao, H.: Delicaflavone induces ROS-mediated apoptosis and inhibits PI3K/AKT/mTOR and Ras/MEK/Erk signaling pathways in colorectal cancer cells. Biochem. Pharmacol. 171, 113680 (2019). https://doi.org/10.1016/j.bcp.2019.113680
Zhong, Y., Jin, C., Gan, J., Wang, X., Shi, Z., Xia, X., Peng, X.: Apigenin attenuates patulin-induced apoptosis in HEK293 cells by modulating ROS-mediated mitochondrial dysfunction and caspase signal pathway. Toxicon. 137, 106–113 (2017). https://doi.org/10.1016/j.toxicon.2017.07.018
Acknowledgments
Thanks to the College of Food Science and Engineering, Tianjin University of Science and Technology for supporting my research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meng, T., Yu, Ss., Ji, Hy. et al. A novel acid polysaccharide from Boletus edulis: extraction, characteristics and antitumor activities in vitro. Glycoconj J 38, 13–24 (2021). https://doi.org/10.1007/s10719-021-09972-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-021-09972-0