Abstract
Significant changes of glycan structures are observed in humans if diseases like cancer, arthritis or inflammation are present. Thus, interest in biomarkers based on glycan structures has rapidly emerged in recent years and monitoring disease specific changes of glycosylation and their quantification is of great interest. Mass spectrometry is most commonly used to characterize and quantify glycopeptides and glycans liberated from the glycoprotein of interest. However, ionization properties of glycopeptides can strongly depend on their composition and can therefore lead to intensities that do not reflect the actual proportions present in the intact glycoprotein. Here we show that an increase in the length of the peptide can lead to a more accurate determination and quantification of the glycans. The four glycosylation sites of human serum ceruloplasmin from 17 different individuals were analyzed using glycopeptides of varying peptide lengths, obtained by action of different proteases and by limited digestion. In most cases, highly sialylated compositions showed an increased relative abundance with increasing peptide length. We observed a relative increase of triantennary glycans of up to a factor of three and, even more, MS peaks corresponding to tetraantennary compositions on ceruloplasmin at glycosite 137N in all 17 samples, which we did not detect using a bottom up approach. The data presented here leads to the conclusion that a middle down - or when possible a top down - approach is favorable for qualitative and quantitative analysis of the glycosylation of glycoproteins.








Similar content being viewed by others
Abbreviations
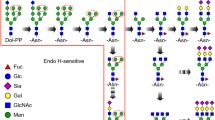
- H:
-
hexose
- N:
-
N-acetylhexosamine
- S:
-
sialic acid
- F:
-
fucose
References
Maverakis, E., Kim, K., Shimoda, M., Gershwin, M.E., Patel, F., Wilken, R., Raychaudhuri, S., Ruhaak, L.R., Lebrilla, C.B.: Glycans in the immune system and the altered glycan theory of autoimmunity: a critical review. J. Autoimmun. 57, 1–13 (2015). https://doi.org/10.1016/j.jaut.2014.12.002
Kenan, N., Larsson, A., Axelsson, O., Helander, A.: Changes in transferrin glycosylation during pregnancy may lead to false-positive carbohydrate-deficient transferrin (CDT) results in testing for riskful alcohol consumption. Clin. Chim. Acta. 412(1), 129–133 (2011). https://doi.org/10.1016/j.cca.2010.09.022
Golka, K., Wiese, A.: Carbohydrate-deficient transferrin (CDT)-a biomarker for long-term alcohol consumption. J. Toxicol. Environ. Health B. 7(4), 319–337 (2004). https://doi.org/10.1080/10937400490432400
Sorvajärvi, K., Blake, J.E., Israel, Y., Niemelä, O.: Sensitivity and specificity of carbohydrate-deficient transferrin as a marker of alcohol abuse are significantly influenced by alterations in serum transferrin: comparison of two methods. Alcohol. Clin. Exp. Res. 20(3), 449–454 (1996). https://doi.org/10.1111/j.1530-0277.1996.tb01074.x
Yin, H., Lin, Z., Nie, S., Wu, J., Tan, Z., Zhu, J., Dai, J., Feng, Z., Marrero, J., Lubman, D.M.: Mass-selected site-specific Core-Fucosylation of Ceruloplasmin in alcohol-related hepatocellular carcinoma. J. Proteome Res. 13(6), 2887–2896 (2014). https://doi.org/10.1021/pr500043k
Balmaña, M., Sarrats, A., Llop, E., Barrabés, S., Saldova, R., Ferri, M.J., Figueras, J., Fort, E., de Llorens, R., Rudd, P.M., Peracaula, R.: Identification of potential pancreatic cancer serum markers: increased sialyl-Lewis X on ceruloplasmin. Clin. Chim. Acta. 442, 56–62 (2015). https://doi.org/10.1016/j.cca.2015.01.007
Bowman, M.J., Zaia, J.: Novel tags for the stable isotopic labeling of carbohydrates and quantitative analysis by mass spectrometry. Anal. Chem. 79(15), 5777–5784 (2007). https://doi.org/10.1021/ac070581b
Zhang, H., Yan, W., Aebersold, R.: Chemical probes and tandem mass spectrometry: a strategy for the quantitative analysis of proteomes and subproteomes. Curr. Opin. Chem. Biol. 8(1), 66–75 (2004). https://doi.org/10.1016/j.cbpa.2003.12.001
Zhang, H., Yi, E.C., Li, X.-j., Mallick, P., Kelly-Spratt, K.S., Masselon, C.D., Camp, D.G., Smith, R.D., Kemp, C.J., Aebersold, R.: High throughput quantitative analysis of serum proteins using Glycopeptide capture and liquid chromatography mass spectrometry. Mol. Cell. Proteomics. 4(2), 144–155 (2005). https://doi.org/10.1074/mcp.M400090-MCP200
Wohlgemuth, J., Karas, M., Eichhorn, T., Hendriks, R., Andrecht, S.: Quantitative site-specific analysis of protein glycosylation by LC-MS using different glycopeptide-enrichment strategies. Anal. Biochem. 395(2), 178–188 (2009). https://doi.org/10.1016/j.ab.2009.08.023
Hofmann, J., Hahm, H.S., Seeberger, P.H., Pagel, K.: Identification of carbohydrate anomers using ion mobility–mass spectrometry. Nature. 526, 241–244 (2015). https://doi.org/10.1038/nature15388
Wuhrer, M., Catalina, M.I., Deelder, A.M., Hokke, C.H.: Glycoproteomics based on tandem mass spectrometry of glycopeptides. J. Chromatogr. B. 849(1), 115–128 (2007). https://doi.org/10.1016/j.jchromb.2006.09.041
Zaia, J.: Mass spectrometry and Glycomics. OMICS. 14(4), 401–418 (2010). https://doi.org/10.1089/omi.2009.0146
Ruhaak, L.R., Miyamoto, S., Lebrilla, C.B.: Developments in the identification of glycan biomarkers for the detection of Cancer. Mol. Cell. Proteomics. 12(4), 846–855 (2013). https://doi.org/10.1074/mcp.R112.026799
Aizpurua-Olaizola, O., Sastre Toraño, J., Falcon-Perez, J.M., Williams, C., Reichardt, N., Boons, G.J.: Mass spectrometry for glycan biomarker discovery. TrAC Trends Anal. Chem. 100, 7–14 (2018). https://doi.org/10.1016/j.trac.2017.12.015
Svarovsky, S.A., Joshi, L.: Cancer glycan biomarkers and their detection – past, present and future. Anal. Methods. 6(12), 3918–3936 (2014). https://doi.org/10.1039/C3AY42243G
Gilgunn, S., Conroy, P.J., Saldova, R., Rudd, P.M., O'Kennedy, R.J.: Aberrant PSA glycosylation—a sweet predictor of prostate cancer. Nat. Rev. Urol. 10, 99–107 (2013). https://doi.org/10.1038/nrurol.2012.258
Ercan, A., Cui, J., Chatterton, D.E.W., Deane, K.D., Hazen, M.M., Brintnell, W., O'Donnell, C.I., Derber, L.A., Weinblatt, M.E., Shadick, N.A., Bell, D.A., Cairns, E., Solomon, D.H., Holers, V.M., Rudd, P.M., Lee, D.M.: Aberrant IgG galactosylation precedes disease onset, correlates with disease activity, and is prevalent in autoantibodies in rheumatoid arthritis. Arthritis Rheum. 62(8), 2239–2248 (2010). https://doi.org/10.1002/art.27533
Han, L., Costello, C.E.: Mass spectrometry of Glycans. Biochemistry. Biokhimiia. 78(7), 710–720 (2013). https://doi.org/10.1134/S0006297913070031
Wada, Y., Azadi, P., Costello, C.E., Dell, A., Dwek, R.A., Geyer, H., Geyer, R., Kakehi, K., Karlsson, N.G., Kato, K., Kawasaki, N., Khoo, K.-H., Kim, S., Kondo, A., Lattova, E., Mechref, Y., Miyoshi, E., Nakamura, K., Narimatsu, H., Novotny, M.V., Packer, N.H., Perreault, H., Peter-Katalinić, J., Pohlentz, G., Reinhold, V.N., Rudd, P.M., Suzuki, A., Taniguchi, N.: Comparison of the methods for profiling glycoprotein glycans—HUPO human disease Glycomics/proteome initiative multi-institutional study. Glycobiology. 17(4), 411–422 (2007). https://doi.org/10.1093/glycob/cwl086
Grünwald-Gruber, C., Thader, A., Maresch, D., Dalik, T., Altmann, F.: Determination of true ratios of different N-glycan structures in electrospray ionization mass spectrometry. Anal. Bioanal. Chem. 409(10), 2519–2530 (2017). https://doi.org/10.1007/s00216-017-0235-8
Walker, S.H., Papas, B.N., Comins, D.L., Muddiman, D.C.: The interplay of permanent charge and hydrophobicity in the electrospray ionization of Glycans. Anal. Chem. 82(15), 6636–6642 (2010). https://doi.org/10.1021/ac101227a
Harvey, D.J.: Quantitative aspects of the matrix-assisted laser desorption mass spectrometry of complex oligosaccharides. Rapid Commun. Mass Spectrom. 7(7), 614–619 (1993). https://doi.org/10.1002/rcm.1290070712
Chen, F.-T.A., Dobashi, T.S., Evangelista, R.A.: Quantitative analysis of sugar constituents of glycoproteins by capillary electrophoresis. Glycobiology. 8(11), 1045–1052 (1998). https://doi.org/10.1093/glycob/8.11.1045
Yamashita, K., Liang, C.J., Funakoshi, S., Kobata, A.: Structural studies of asparagine-linked sugar chains of human ceruloplasmin. Structural characteristics of the triantennary complex type sugar chains of human plasma glycoproteins. J. Biol. Chem. 256(3), 1283–1289 (1981)
Endo, M., Suzuki, K., Schmid, K., Fournet, B., Karamanos, Y., Montreuil, J., Dorland, L., van Halbeek, H., Vliegenthart, J.F.: The structures and microheterogeneity of the carbohydrate chains of human plasma ceruloplasmin. A study employing 500-MHz 1H-NMR spectroscopy. J. Biol. Chem. 257(15), 8755–8760 (1982)
Bielli, P., Calabrese, L.: Structure to function relationships in ceruloplasmin: a 'moonlighting' protein. Cell. Mol. Life Sci. 59(9), 1413–1427 (2002). https://doi.org/10.1007/s00018-002-8519-2
Sogabe, M., Nozaki, H., Tanaka, N., Kubota, T., Kaji, H., Kuno, A., Togayachi, A., Gotoh, M., Nakanishi, H., Nakanishi, T., Mikami, M., Suzuki, N., Kiguchi, K., Ikehara, Y., Narimatsu, H.: Novel Glycobiomarker for ovarian Cancer that detects clear cell carcinoma. J. Proteome Res. 13(3), 1624–1635 (2014). https://doi.org/10.1021/pr401109n
Harazono, A., Kawasaki, N., Itoh, S., Hashii, N., Ishii-Watabe, A., Kawanishi, T., Hayakawa, T.: Site-specific N-glycosylation analysis of human plasma ceruloplasmin using liquid chromatography with electrospray ionization tandem mass spectrometry. Anal. Biochem. 348(2), 259–268 (2006). https://doi.org/10.1016/j.ab.2005.10.036
Burkhart, J.M., Schumbrutzki, C., Wortelkamp, S., Sickmann, A., Zahedi, R.P.: Systematic and quantitative comparison of digest efficiency and specificity reveals the impact of trypsin quality on MS-based proteomics. J. Proteome. 75(4), 1454–1462 (2012). https://doi.org/10.1016/j.jprot.2011.11.016
Bento, I., Peixoto, C., Zaitsev, V.N., Lindley, P.F.: Ceruloplasmin revisited: structural and functional roles of various metal cation-binding sites. Acta Crystallogr. D Biol. Crystallogr. 63(Pt 2), 240–248 (2007). https://doi.org/10.1107/S090744490604947X
Kyte, J., Doolittle, R.F.: A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157(1), 105–132 (1982). https://doi.org/10.1016/0022-2836(82)90515-0
Clerc, F., Reiding, K.R., Jansen, B.C., Kammeijer, G.S.M., Bondt, A., Wuhrer, M.: Human plasma protein N-glycosylation. Glycoconj. J. 33(3), 309–343 (2016). https://doi.org/10.1007/s10719-015-9626-2
Guthals, A., Bandeira, N.: Peptide identification by tandem mass spectrometry with alternate fragmentation modes. Mol. Cell. Proteomics. 11(9), 550–557 (2012). https://doi.org/10.1074/mcp.r112.018556
Huang, Y., Triscari, J.M., Tseng, G.C., Pasa-Tolic, L., Lipton, M.S., Smith, R.D., Wysocki, V.H.: Statistical characterization of the charge state and residue dependence of low-energy CID peptide dissociation patterns. Anal. Chem. 77(18), 5800–5813 (2005). https://doi.org/10.1021/ac0480949
Harrison, A.G.: Energy-resolved mass spectrometry: a comparison of quadrupole cell and cone-voltage collision-induced dissociation. Rapid Commun. Mass Spectrom. 13(16), 1663–1670 (1999). https://doi.org/10.1002/(SICI)1097-0231(19990830)13:16<1663::AID-RCM695>3.0.CO;2-T
Hiroshi, M., Toshifumi, T., Yasutsugu, S., Takekiyo, M.: Optimization of skimmer voltages of an electrospray ion source coupled with a magnetic sector instrument. Rapid Commun. Mass Spectrom. 8(2), 205–210 (1994). https://doi.org/10.1002/rcm.1290080216
Lin, C.-H., Krisp, C., Packer, N.H., Molloy, M.P.: Development of a data independent acquisition mass spectrometry workflow to enable glycopeptide analysis without predefined glycan compositional knowledge. J. Proteome. 172, 68–75 (2018). https://doi.org/10.1016/j.jprot.2017.10.011
Zacchi, L.F., Schulz, B.L.: SWATH-MS Glycoproteomics reveals consequences of defects in the glycosylation machinery. Mol. Cell. Proteomics. 15(7), 2435–2447 (2016). https://doi.org/10.1074/mcp.M115.056366
Yeo, K.Y.B., Chrysanthopoulos, P.K., Nouwens, A.S., Marcellin, E., Schulz, B.L.: High-performance targeted mass spectrometry with precision data-independent acquisition reveals site-specific glycosylation macroheterogeneity. Anal. Biochem. 510, 106–113 (2016). https://doi.org/10.1016/j.ab.2016.06.009
Tsou, C.-C., Avtonomov, D., Larsen, B., Tucholska, M., Choi, H., Gingras, A.-C., Nesvizhskii, A.I.: DIA-umpire: comprehensive computational framework for data-independent acquisition proteomics. Nat. Methods. 12, 258–264 (2015). https://doi.org/10.1038/nmeth.3255
MacLean, B., Tomazela, D.M., Shulman, N., Chambers, M., Finney, G.L., Frewen, B., Kern, R., Tabb, D.L., Liebler, D.C., MacCoss, M.J.: Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. 26(7), 966–968 (2010). https://doi.org/10.1093/bioinformatics/btq054
Gillet, L.C., Navarro, P., Tate, S., Röst, H., Selevsek, N., Reiter, L., Bonner, R., Aebersold, R.: Targeted data extraction of the MS/MS spectra generated by data-independent acquisition: a new concept for consistent and accurate proteome analysis. Mol. Cell. Proteomics. 11(6), O111.016717 (2012). https://doi.org/10.1074/mcp.O111.016717
Yang, Y., Wang, G., Song, T., Lebrilla, C.B., Heck, A.J.R.: Resolving the micro-heterogeneity and structural integrity of monoclonal antibodies by hybrid mass spectrometric approaches. mAbs. 9(4), 638–645 (2017). https://doi.org/10.1080/19420862.2017.1290033
Sinha, S., Pipes, G., Topp, E.M., Bondarenko, P.V., Treuheit, M.J., Gadgil, H.S.: Comparison of LC and LC/MS methods for quantifying N-glycosylation in recombinant IgGs. J. Am. Soc. Mass Spectrom. 19(11), 1643–1654 (2008). https://doi.org/10.1016/j.jasms.2008.07.004
Tipton, J.D., Tran, J.C., Catherman, A.D., Ahlf, D.R., Durbin, K.R., Kelleher, N.L.: Analysis of intact protein isoforms by mass spectrometry. J. Biol. Chem. 286(29), 25451–25458 (2011). https://doi.org/10.1074/jbc.R111.239442
Hochstrasser, H., Bauer, P., Walter, U., Behnke, S., Spiegel, J., Csoti, I., Zeiler, B., Bornemann, A., Pahnke, J., Becker, G., Riess, O., Berg, D.: Ceruloplasmin gene variations and substantia nigra hyperechogenicity in Parkinson disease. Neurology. 63(10), 1912–1917 (2004). https://doi.org/10.1212/01.wnl.0000144276.29988.c3
Hochstrasser, H., Tomiuk, J., Walter, U., Behnke, S., Spiegel, J., Krüger, R., Becker, G., Riess, O., Berg, D.: Functional relevance of ceruloplasmin mutations in Parkinson’s disease. FASEB J. 19(13), 1851–1853 (2005). https://doi.org/10.1096/fj.04-3486fje
Leymarie, N., Griffin, P.J., Jonscher, K., Kolarich, D., Orlando, R., McComb, M., Zaia, J., Aguilan, J., Alley, W.R., Altmann, F., Ball, L.E., Basumallick, L., Bazemore-Walker, C.R., Behnken, H., Blank, M.A., Brown, K.J., Bunz, S.-C., Cairo, C.W., Cipollo, J.F., Daneshfar, R., Desaire, H., Drake, R.R., Go, E.P., Goldman, R., Gruber, C., Halim, A., Hathout, Y., Hensbergen, P.J., Horn, D.M., Hurum, D., Jabs, W., Larson, G., Ly, M., Mann, B.F., Marx, K., Mechref, Y., Meyer, B., Möginger, U., Neusüβ, C., Nilsson, J., Novotny, M.V., Nyalwidhe, J.O., Packer, N.H., Pompach, P., Reiz, B., Resemann, A., Rohrer, J.S., Ruthenbeck, A., Sanda, M., Schulz, J.M., Schweiger-Hufnagel, U., Sihlbom, C., Song, E., Staples, G.O., Suckau, D., Tang, H., Thaysen-Andersen, M., Viner, R.I., An, Y., Valmu, L., Wada, Y., Watson, M., Windwarder, M., Whittal, R., Wuhrer, M., Zhu, Y., Zou, C.: Interlaboratory study on differential analysis of protein glycosylation by mass spectrometry: the ABRF glycoprotein research multi-institutional study 2012. Mol. Cell. Proteomics. 12(10), 2935–2951 (2013). https://doi.org/10.1074/mcp.M113.030643
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Baerenfaenger, M., Moritz, M. & Meyer, B. Quantitation of Glycopeptides by ESI/MS - size of the peptide part strongly affects the relative proportions and allows discovery of new glycan compositions of Ceruloplasmin. Glycoconj J 36, 13–26 (2019). https://doi.org/10.1007/s10719-018-9852-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-018-9852-5