Abstract
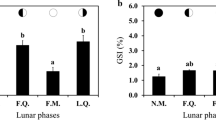
Testicular development and plasma levels of sex steroid [11-ketotestosterone (11-KT), testosterone (T) and 17,20β-dihydoxy-4-pregnen-3-one (17,20β-P)] were studied for the first time in wild golden mahseer, Tor putitora. Testicular development was investigated by macroscopic observation and histology of the gonads, whereas steroids were measured by enzyme-linked immunosorbent assay. Based on macroscopic observation and germ cell types present in gonad histology, the testes of T. putitora were divided into five developmental stages: immature [stage I; spermatogonia (SPG)], early spermatogenesis [stage II; SPG and spermatocytes (SPC)], late spermatogenesis [stage III; SPG, SPC, spermatids (SPD) and spermatozoa (SPZ)], spermiation (stage IV; SPZ) and post-spawning (stage V; SPG, SPD and SPZ). During the stage I of the testes, the lowest levels of plasma sex steroid and gonadosomatic index (I G) were recorded. The highest plasma level of T was 0.89 ± 0.09 ng/mL and 11-KT was 4.23 ± 0.54 ng/mL, which was during the stage III and IV, respectively. The peak in 11-KT was coincident with the peak in I G (1.65 ± 0.12 %). The lowest T and 11-KT levels were 0.25 ± 0.02 ng/mL and 0.47 ± 0.09 ng/mL, respectively, which was at stage I. Plasma levels of 17,20β-P increased significantly at stage III (1.04 ± 0.06 ng/mL) and stage IV testes (1.28 ± 0.03 ng/mL) and then declined in post-spawned fish. This indicates that 17,20β-P could also be a possible maturation-inducing steroid in this fish. The condition factor (K) significantly decreased during the testicular development and was lowest at spermiation stage (0.53 ± 0.02 %). The proportion of running male peaked concomitantly with the appearance of stage IV testes. Presence of germ cells of different developmental stages indicates that T. putitora male is a multiple spawner, and the information generated here is important for developing a captive breeding, culture and conservation programs for this endangered coldwater Himalayan fish species.





Similar content being viewed by others
References
Adebiyi FA, Siraj SS, Harmin SA, Christianus A (2013) Plasma sex steroid hormonal profile and gonad histology during the annual reproductive cycle of river catfish Hemibagrus nemurus (Valenciennes, 1840) in captivity. Fish Physiol Biochem 39:547–557
Allen TC (1993) Haematoxylin and eosin. In: Phophet EB, Mills B, Arrington JB, Sobin MD (eds) Laboratory methods in histotechnology. American Registry of Pathology, Washington, pp 53–58
Aramli MS, Kalbassi MR, Nazari RM (2014) Sex steroid levels of Persian sturgeon, Acipenser persicus, Borodin, 1897, males in negative and positive responding to LH–RH-analogue. J Appl Ichthyol 30:18–19
Baynes SM, Scott AP (1985) Seasonal variations in parameters of milt production and in plasma concentration of sex steroids of male rainbow trout (Salmo gairdneri). Gen Comp Endocrinol 57:150–160
Bhatt JP, Nautiyal P, Singh HR (2000) Population structure of Himalayan mahseer, a large cyprinid fish in the regulated foothill section of the river Ganga. Fish Res 44:267–271
Billard R, Fostier A, Weil C, Breton B (1982) Endocrine control of spermatogenesis in Teleost fish. Can J Fish Aquat Sci 39:65–79
Borg B (1994) Androgens in teleost fishes. Comp Biochem Physiol 109C:219–245
Canario AVM (1991) Sex steroids in marine flatfish. In: Scott AP, Sumpter JP, Kime DE, Rolfe MS (eds) Proceedings of the fourth international symposium on the reproductive physiology of fish. FishSymp91, Sheffield pp 224–234
Canario AVM, Scott AP (1990) Effects of steroids and human chorionic gonadotropin on in vitro oocyte final maturation in two marine flatfish: the dab, Limanda limanda, and the plaice, Pleuronectes platessa. Gen Comp Endocrinol 77:161–176
Cavaco JEB, Vischer HF, Lambert JGD, Goos HJTh, Schulz RE (1997a) Mismatch between patterns of circulating and testicular androgens in African catfish, Clarias gariepinus. Fish Physiol Biochem 17:155–162
Cavaco JEB, Lambert JGD, Schulz RW, Goos HJTh (1997b) Pubertal development of male African catfish, Clarias gariepinus. In vitro steroidogenesis by testis and interregnal tissue and plasma levels of sexual steroids. Fish Physiol Biochem 16:129–138
Chaves-Pozo E, Arjona FJ, García-López A, García-Alcázar A, Meseguer J, García-Ayala A (2008) Sex steroids and metabolic parameter levels in a seasonal breeding fish (Sparus aurata L.). Gen Comp Endocrinol 156:531–536
Cuisset B, Pradelles P, Kime DE, Kuhn ER, Babin P, Le Menn F (1994) Enzyme immune assay for 11-ketotestosterone using acetylcholinesterase as label. Application to measurement of 11-ketotestosterone in plasma of Siberian sturgeon. Comp Biochem Phys C 108:229–241
De Rooij DG (2006) Regulation of spermatogonial stem cells behaviour in vivo and in vitro. Anim Reprod 3:130–134
Degani G, Boker R, Jackson K (1998) Growth hormone, sexual maturity and steroids in male carp (Cyprinus carpio). Comp Biochem Phys C 120:433–440
Dinesh K, Nandeesha MC, Nautiyal P, Aiyappa P (2010) Mahseers in India: a review with focus on conservation and management. Indian J Anim Sci 80:26–38
Estay F, Diaz A, Pendrazza R, Colihueque N (2003) Oogenesis and plasma levels of sex steroids in cultured females of brown trout (Salmo trutta, Linnaeus, 1758) in Chile. J Exp Zool 298A:60–66
Fostier A, Jalabert B, Billard R, Breton B, Zohar Y (1983) The gonadal steroids. In: Hoar WS, Randall DJ, Donaldson EM (eds) Fish physiology IX A. Academic Press, New York, pp 277–345
Froese R, Pauly D (Ed) (2011) Fishbase. World Wide Web electronic publication. http://fishbase.org. Accessed 17 April 2014
García-López Ά, Fernández-Pasquier V, Couto E, Canario AVM, Sarasquete C, Martínez-Rodríguez G (2006) Testicular development and plasma sex steroid levels in cultured male Senegalese sol Solea senegalensis Kaup. Gen Comp Endocrinol 147:343–351
Gurgel HCB (2004) Population structure and breeding season of Astyanax fasciatus (Cuvier) (Characidae, Tetragonopterinae) from Ceara Mirim River, Poco Branco, Rio Grande do Norte, Brazil. Rev Bras Zool 21:131–135
Harmin SA, Crim LW, Wiegand MD (1995) Plasma sex steroid profiles and the seasonal reproductive cycle in male and female winter flounder, Pleuronectes americanus. Mar Biol 121:601–610
Ingram B, Sungan S, Gooley G, Sim SY, Tinggi D, De Silva S (2005) Induced spawning, larval rearing of two indigenous Malaysia Mahseer, Tor tromboides and Tor douronensis. Aquac Res 36:1001–1014
Islam SM, Tanaka M (2007) Threatened fishes of the world: Tor putitora Hamilton 1822 (Cypriniformes: Cyprinidae). Environ Biol Fish 78:219–220
Ismail MFS, Siraj SS, Daud SK, Harmin SA (2011) Association of annual hormonal profile with gonad maturity of mahseer (Tor tambroides) in captivity. Gen Comp Endocrinol 170:125–130
Jayaram KC (2010) The freshwater fishes of Indian region. Narendra Publishing House, New Delhi
Koya Y, Soyano K, Yamamoto K, Obana H, Matsubara T (2002) Testicular development and serum profiles of steroid hormone levels in captive male Pacific herring Clupea pallasii during their first maturational cycle. Fish Sci 68:1099–1105
Koya Y, Watanabe H, Soyano K, Ohta K, Aritaki M, Matsubara T (2003) Testicular development and serum steroid hormone levels in captive male spotted halibut Verasper variegates. Fish Sci 69:792–798
Lee WK, Yang SW (2002) Relationship between ovarian development and serum levels of gonadal steroid hormones and induction of oocyte maturation and ovulation in the cultured female Korean spotted sea bass Lateolabrax maculates (Jeom-nongoeo). Aquaculture 207:169–183
Lintelmann J, Katayama A, Kurihara N, Shore L, Wenzel A (2003) Endocrine disruptors in the environment. Pure Appl Chem 75:631–681
Liu HW, Stickney RR, Dickhoff WW (1991) Changes in plasma concentrations of sex steroids in adult Pacific halibut, Hippoglossus stenolepis. J World Aquac Soc 22:30–35
Matsuyama M, Adachi S, Nagahama Y, Kitajima C, Matsuura S (1991) Testicular development and serum levels of gonadal steroids during the annual reproductive cycle of captive Japanese sardine. Jpn J Ichthyol 37:381–390
Mayer I, Borg B, Schulz R (1990) Conversion of 11-ketoandrostenedione to 11-ketotestosterone by blood cells of six fish species. Gen Comp Endocrinol 77:70–74
Miura T, Yamauchi K, Takahashi H, Nagahama Y (1991) Hormonal induction of all stages of spermatogenesis in vitro in the male Japanese eel (Anguilla japonica). Proc Natl Acad Sci USA 88:5774–5778
Miura T, Yamauchi K, Takahashi T, Nagahama Y (1992) The role of hormones in the acquisition of sperm mobility in salmonid fish. J Exp Zool 261:359–363
Nagahama Y (1994) Endocrine regulation of gametogenesis in fish. Int J Dev Biol 38:217–229
Nash JP, Davail-Cuisset B, Bhattacharyya S, Suter H, Le Menn F, Kime DE (2000) An enzyme linked immunosorbent assay (ELISA) for testosterone, 17β-estradiol and 17α,20β-dihydroxy-4-pregnen-3-one using acetylcholinesterase as tracer: application to measurement of diel patterns in rainbow trout (Oncorhynchus mykiss). Fish Physiol Biochem 22:355–363
Nautiyal P (1984) Natural history of the Garhwal Himalayan mahseer Tor putitora (Hamilton) breeding biology. Proc Anim Sci 93:97–106
Nikolsky GV (1963) Ecology of fishes. Academic Press, London, p 352
Oliver K, Sangma V, Basavaraja N (2007) Deccan Mahseer (Tor khudree) of Karnataka-on location of its wild brooders and fry and breakthrough in the hatchery production of its seed. Fish Chim 26:32–36
Pandey AK, Patiyal RS, Upadhyay JC, Tyagi M, Mahanta PC (1998) Induced spawning of the endangered golden mahseer, Tor putitora, with ovaprim at the state fish farm near Dehradun. Indian J Fish 45:457–459
Rinchard J, Kestemont P, Heine R (1997) Comparative study of reproductive biology in single and multiple spawner cyprinid fish. II. Sex steroid and plasma protein phosphorus concentrations. J Fish Biol 50:169–180
Rosenblum PM, Pudney J, Callard IP (1987) Gonadal morphology, enzyme histochemistry and plasma steroid levels during the annual reproductive cycle of male and female brown bullhead catfish, Ictalurus nebulosus Lesueur. J Fish Biol 31:325–341
Santana JCO, Quagio-Grassiotto I (2014) Extracellular matrix remodelling of the testes through the male reproductive cycle in Teleostei fish. Fish Physiol Biochem. doi:10.1007/s10695-014-9974-z
Schulz RW (2003) Endocrine regulation of spermatogenesis in teleost fish. ARBS Annu Rev Biomed Sci 5:57–68
Schulz RW, Miura T (2002) Spermatogenesis and its endocrine regulation. Fish Physiol Biochem 26:43–56
Schulz RW, de Franca LR, Lareyre JJ, LeGac F, Garcia HC, Nobrega RH, Miura T (2010) Spermatogenesis in fish. Gen Comp Endocrinol 165:390–411
Scott AP, Canario AV (1992) 17α,20β-Dihydroxi-4-pregnon-3-one 20 sulphate: a major new metabolite of the teleost oocyte maturation inducing steroid. Gen Comp Endocrinol 85:91–100
Scott AP, Bye VJ, Baynes SM, Springate JRC (1980) Seasonal variations in plasma concentrations of 11-ketotestosterone and testosterone in male rainbow trout, Salmo gairdnerii Richardson. J Fish Biol 17:495–505
Shinkafi BA, Ipinjolu JK, Hassan WA (2011) Gonad maturation stages of Auchenoglanis occidentalis (Valenciennes 1840) in River Rima, North-Western Nigeria. J Fish Aquat Sci 6:236–246
Sol SY, Olson OP, Lomax DP, Johnson LL (1998) Gonadal development and associated changes in plasma reproductive steroids in English sol, Pleuronectes vetulus, from Puget Sound, Washington. Fish B NOAA 96:859–870
Vermeulen GJ, Lambert JGD, Teitsma CA, Zandbergen MA, Goos HJTh (1995) Adrenal tissue in the male African catfish, Clarias gariepinus: localization and steroid hormone secretion. Cell Tissue Res 280:653–657
Weltzien FA, Taranger GL, Karlsen Ø, Norberg B (2002) Spermatogenesis and related plasma androgen levels in Atlantic halibut (Hippoglossus hippoglossus L.). Comp Biochem Phys A 132:567–575
Yueh WS, Chang CF (1997) 17α,20β,21-Trihydroxy-4-pregnen-3-one and 17α,20β-dihydroxy-4-pregnen-3-one stimulated spermiation in protandrous black porgy, Acanthopagrus schlegeli. Fish Physiol Biochem 17:187–193
Acknowledgments
This research was supported by a project grant from Indian Council of Agricultural Research (ICAR), New Delhi, India, to first author (NS). The authors acknowledge Monalisa Sahoo of Indian Veterinary Research Institute (IVRI), Bareilly, for her assistance in histological preparations and data analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shahi, N., Mallik, S.K., Pande, J. et al. Spermatogenesis and related plasma androgen and progestin level in wild male golden mahseer, Tor putitora (Hamilton, 1822), during the spawning season. Fish Physiol Biochem 41, 909–920 (2015). https://doi.org/10.1007/s10695-015-0057-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-015-0057-6