Abstract
To protect ancient buildings from fire, prevent the occurrences of fire, and minimise the losses caused by fire to the maximum extent possible, this study combined experimental measurements and numerical simulations to analyse the flame spread behaviour over wood treated with flame retardants. First, some wood blocks were treated with a nitrogen and phosphorus (water-based) flame retardant, and then a smoke combustion experiment was performed to test the smoke density. Scanning electron microscopy was also employed to observe the flame retardant effect. Next, a fire dynamic simulation software was used to simulate and analyse the results of the flame spread behaviour over the yellow pine collected from the Long’en Hall of Fuling Mausoleum, that was treated with flame retardants. The results showed the variation trend of the fire site’s heat release rate (HRR) over time to be consistent with the movement of fire spread after its occurrence. Compared with pristine wood, the flame retardant-treated wood exhibited a HRR reduction of 53.1%. The addition of flame retardants also reduced the concentration of the released smoke and CO2 gas, decreased the temperature of the fire site, and enhanced visibility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Many ancient buildings, both indigenous and foreign, have been destroyed by fire. China’s ancient buildings are brick and wood structures with hundreds of years of history, and their structural systems suffer from erosion and weathering. The moisture content of wood is generally very low, which makes it easy to burn [1, 2], and once a fire occurs, it spreads quickly. More than 40% of the cases of fire in ancient buildings occur due to the negligent use of flammable substances such as cotton, hemp, silk, and wool fabrics [3, 4]. In particular, items such as tablecloths, draperies, streamers, and flags are abundant in ancient buildings. Once these fabrics get ignited, they often quickly become the channel for fire transmission [5, 6]. Except for a few ancient buildings located in urban areas, the vast majority are built in suburbs and mountainous regions, which are difficult to access even by foot, let alone by fire trucks [7].
Ancient buildings are usually home to many precious objects such as murals, paintings, artworks, religious objects, etc. Many of these objects and their uses represent danger in their own right (liquids kept in natural history museums, fabrics in textile museums, candles in churches, etc.) [8,9,10]. These features of ancient buildings make them challenging because it is usually unacceptable to alter their structure to include modern fire protection features (ex., smoke detectors, sprinklers, fire-rated stairs, etc.) [11, 12]. Moreover, it is often impossible to manage hazards in a manner consistent with code assumptions (e.g., no combustibles in egress paths, fuel characteristics corresponding to the assumptions embedded in the design of fire protection systems, etc.). Thus, the fire safety design of a historic building represents a significant challenge, which involves several alteration constraints and the unsuitability of standard fire protection tools [13].
Considerable research effort has been devoted to treating wood with flame retardants [14,15,16]. Conventional flame retardant methods generally involve soaking wood with flame retardants or applying flame-resistant coatings to wooden surfaces [17,18,19,20,21]. Halogen and phosphorus-based flame retardants are primarily regarded as the most effective coatings to prevent wood from igniting [22]. Although this kind of flame retardant has many shortcomings [23, 24], it is currently the most effective flame retardant for wood in ancient Chinese buildings.
Fuling Mausoleum is located in the eastern suburbs of Shenyang City, Liaoning Province, China. Its main ancient buildings are grand in scale, with the overall architectural complex relatively intact. In 2004, Fuling Mausoleum was listed as a World Cultural Heritage site [25, 26]. The Fuling Mausoleum Square City is located at the centre of the mausoleum area. It has a square perimeter of 370 m and a wall of about 5 m high. The width of the entrance and exit holes of Square City is only 2.5 m [27]. All the main buildings of the mausoleum are located within Square City. The primary structures of these ancient buildings are mostly wood and arranged in groups. Their lower parts are mainly supported by an elevated platform, while wooden columns and beams are used to support the enormous roof. The components of the roof, such as brackets, beams, columns, rafters, watch-boards, etc., are mostly made of wood (Fig. 1a). Due to the wall barrier, fire trucks cannot directly enter the inner city of Fuling Mausoleum, which is unconducive to the fire safety of its buildings. Fuling Mausoleum suffered a major fire in 1962, after which its Daming Pavilion was only left with the residual masonry base, with all the wood materials burned out (Fig. 1b). It took 20 years until the end of 1982 to complete its reconstruction and rehabilitation [28].
This study combined experimental measurements and numerical simulations to analyse the flame spread behaviour over wood treated with flame retardants. First, some wood blocks were treated with a nitrogen and phosphorus (water-based) flame retardant, and then a smoke combustion experiment was performed to test the smoke density. Scanning electron microscopy (SEM) was also employed to observe the flame retardant effect. Next, fire dynamics simulator (FDS) software was used to simulate and analyse the results of the flame spread behaviour over the yellow pine collected from the Long’en Hall of Fuling Mausoleum. The underlying danger of this fire safety approach was analysed according to the change rule of heat release rate, spread process of fire and smoke, and change in temperature, CO2 concentration and visibility distribution with time. Based on the analysis, we could control the spread of fire, reduce the loss of cultural relics to the maximum extent, and propose targeted fire prevention measures in the future.
2 Experimental Methods of Wood Treated with Flame Retardants
2.1 Flame Retardant Treatment and Mechanism
Wood was the primary building material in ancient Chinese architecture, and the structures of ancient buildings in north China were mainly built of yellow pine. Flame retardants can increase the ignition temperature of wood and slow down their burning rate. Wooden materials are often treated with fire retardants to effectively delay the combustion process of wood fibre by altering their method of pyrolysis, thereby improving their combustion performance and fire resistance.
Nitrogen and phosphorus (water-based) flame retardants are mainly composed of 85% phosphoric acid, urea, catalyst, and melamine. Their molar ratio of urea, phosphoric acid, and melamine is 2.53:3.2:0.025, and the treated wood properties meet the requirements of the American “BOCA National Building Code” [29]. We selected the yellow pine, commonly used in ancient buildings, and conducted experiments with six wooden blocks of size 100 × 100 × 10 mm each. Amongst them, three blocks were soaked in a nitrogen and phosphorus (water-based) flame retardant for 2 h and then dried. The remaining three blocks were left untreated. The appearance and colour of the substrates treated with the water-based flame retardant did not change significantly, and there was no visible solid residue or prominent spot on their surface (Fig. 3c and f).
The working mechanism of the nitrogen and phosphorus (water-based) flame retardant involved dehydrating, charcoal-forming, and gaseous phases (Fig. 2). The flame retardant’s structure was stable, colourless, and tasteless, and it could quickly form an expanding carbon layer to prevent combustion in the event of a fire. It was a highly efficient halogen-free flame retardant. (1) Dehydrating phase: when the fire was lit, the ignition source enhanced the decomposition temperature. Local degradation: both the flammable gases emitted by pyrolysis and resultant charcoal increased. (2) Charcoal-forming phase: charcoal was the post-pyrolysis material and could thus decrease the formation of flammable items. The flammable gases comprised both small molecules and high energy free radicals that could interfere with the flame chemistry (ignition temperature, etc.). (3) Gaseous phase: when air (oxygen) was supplied, the flame retardant could reduce access to oxygen or cause flame dilution. The combusting zone involved exothermic radical chain reactions between the fuel, oxygen, and high-energy free radicals, potentially forming flames, H2O, and incomplete CO2 combustion products and releasing heat.
2.2 Experiment of Flue Gas Combustion
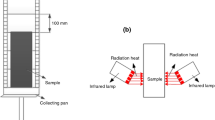
The NES 713 smoke toxicity test chamber (Phoenix, Suzhou, China) and Testo350 flue gas analyser (Testo, Germany) were selected as the laboratory facilities (Fig. 3a and b). Developed as a part of the “NES 713 Determination of the Toxicity index of the products of combustion from small specimens of materials (UK Ministry of Defence, Naval Engineering Standard)” [30] and “GB/T 8627 Test method for density of smoke from the burning or decomposition of building materials” [31], NES 713, at its core, is a test that determines how toxic a specimen is in comparison to other known materials’ toxicity levels. To accomplish precise molecular measurements from small specimens, a sample is thoroughly burned in a control chamber containing adequate amounts of air. NES 713 is capable of capturing results that allow for the comparison of both synthetic and natural test materials and is an apt test for professionals tasked with quality control and/or research and development of materials used in naval equipment. The toxicity index is a numerical summation of the toxicity factors of the individual gases produced during the process of total combustion of a test specimen under controlled conditions. The toxicity factors are determined by calculating the quantity of a specific gas produced as the result of burning 100 g of the test material in a volume of one cubic meter of air.
The experiment of flue gas combustion. (a) Flame images of the wood material without flame retardants. (b) Flame images of the wood material with flame retardants. (c–e) Yellow pine without flame retardants: (c) The front side of the block before combustion. (d) The front side of the block after combustion. (e) The back side of the block after combustion. (f–h) Yellow pine treated with flame retardants: (f) The front side of the block before combustion. (g) The front side of the block after combustion. (h) The back side of the block after combustion
The combustion experiments on yellow pine, both pristine and treated with flame retardants, were performed. The heat release rate per unit area (HRRPUA) of the fire source was set at 1500 KW/m2. Each woodblock lasted for 5 min. The experiment investigated the toxic gases produced when a small sample material was completely burned under specific conditions and analysed their density. During the investigation, the combustion furnace of the NES 713 smoke toxicity test chamber was preheated to 800 °C, and six woodblocks were burned separately. Various combustion gases were collected by using the airflow emission rate. The quantity of each substance released by combustion was then calculated through chemical analysis, and its toxicity was expressed numerically. Lastly, the average of the collected data was taken. The Testo350 flue gas analyser was based on six flue gas sensors, CO, CO2, NO, NO2, SO2, and NOX, and analysed the smoke density.
2.3 SEM Observation
After performing the combustion experiments, each wooden block was cut into a size of 20 × 20 × 10 mm. The Hitachi S-3400 N scanning electron microscope was selected. Its magnification was set at 100, 400, and 1000 times to observe the SEM images of sizes 200, 50, and 20 μm, respectively, of the samples (Fig. 4) and analyse the effect of flame retardants.
SEM observation. (a–c) SEM images of yellow pine without flame retardants before combustion: (a) 200 μm. (b) 50 μm. (c) 20 μm. (d–f) SEM images of yellow pine without flame retardants after combustion: (d) 200 μm. (e) 50 μm. (f) 20 μm. (g–i) SEM images of yellow pine treated with flame retardants after combustion: (g) 200 μm. (h) 50 μm. (i) 20 μm
3 Experimental Results and Discussion of Pristine Wood and That Treated with Flame Retardants
3.1 Results and Discussion After Combustion Experiment
The woodblock of pristine yellow pine formed continuous combustion and burned completely with an open flame in the 5-min combustion process (Fig. 3a). In contrast, after burning for 5 min, the woodblock treated with flame retardant in the combustion process had been smouldering and it did not form an open flame, indicating an evident effect of the flame retardant on yellow pine (Fig. 3b). Most areas of the woodblock of pristine yellow pine had been burned away, and the wood material had been charred. The depth of burning erosion ran through the whole block, which splitted into two parts (Fig. 3c–e). The burning erosion area of the woodblock treated with flame retardant was only part of the front side of it. The depth of burning erosion was only half as thick as the woodblock (Fig. 3f–h).
The flue gas concentration data analysed by the experimental equipment are presented in Table 1. As per Table 1, in the combustion process, one woodblock of 100 × 100 × 10 mm produced a large amount of toxic CO gas, i.e., up to 2,478.8 mg/m3. The CO produced by the flame retardant-treated woodblocks was lower by 24.9%. Their percentage of CO2 released also decreased by 9.6%. As the flame retardant contained nitrogen, the amount of NO, NO2, and NOX gases released increased. The amount of SO2 released did not change much.
3.2 Results and Discussion of SEM Observation
As shown in Fig. 4a–c, the wood structure of the pristine yellow pine was dense prior to combustion, and its texture was compact. As shown in Fig. 4d–f, the pristine yellow pine woodblocks burned completely. Their residual carbon structure was relatively loose, with less white wood ashes and many incomplete shapes of residual carbon left on the surface. As shown in Fig. 4g–i, the remaining yellow pine woodblocks treated with flame retardants left abundant residual white wood ashes. There were incomplete carbon residues of various shapes in different degrees of combustion. There were many oxidation states in the phosphorus-containing compounds of the nitrogen and phosphorus flame retardant. Its thermal decomposition products had a strong dehydration effect. Therefore, the polymer surface was covered by carbonisation. The formed carbon film played a flame retardant role. It effectively isolated the heat transfer, protected the internal wood structure from decomposition, prevented combustible gas generation, and ultimately reduced the internal flammability and oxygen heat contact to terminate combustion. The nitrogen and phosphorus flame retardants were very practical for use in the wood of ancient buildings. It effectively safeguarded the structure and strength of the wood and reduced their ignition risk in case of a fire.
4 Numerical Simulation Method of Fire Combustion
Because it is difficult to burn a full-scale model of the building, we can only burn small pieces of wood to verify the effects of flame retardants. These seem that we cannot solve all problems by experimental equipment alone. FDS can provide valuable insight into how a fire may have developed. The ability of the FDS model to predict accurately the temperature and velocity of fire gases has been previously evaluated by conducting experiments, both labscale and fullscale, and measuring quantities of interest [32]. Therefore, we study the effect of flame retardants in the whole building combustion process by numerical simulation method [33, 34]. Then, we can combine the experimental measurements to verify the reliability of the simulation results of a whole building [35].
4.1 FDS Theory
The FDS is a software tool developed by the U.S. National Bureau of Standards and Technology. It can accurately simulate the occurrence of a fire and the corresponding flow of smoke using the basic equations of fluid mechanics. It includes the mass conservation equation, momentum conservation equation, energy conservation equation, component conservation equation, etc. [36, 37]. The pyrolysis model of the conducted combustion experiment was simulated according to the guidance provided by the simulation software [38].
4.2 Calculation Area and Boundary Conditions
Long’en Hall is the most important building of Fuling Mausoleum. The sacrificial table inside the building is a fire hazard because of the incendiary censers and highly flammable curtains on the altar table (Fig. 5a). The fire simulation experiments were carried out on the altar table. First, the FDS software was used to build the model of Long’en Hall (Fig. 5b). The calculation limits were set to a rectangular parallelepiped area (15 × 15 × 5 m). All the structural components inside the ancient buildings were made of yellow pine, and Table 2 shows the differences in the property parameters of the wooden materials treated with the flame retardant and those untreated [39]. All these aspects were contained in the input file of FDS [40].
The simulated fire started on the front altar table (coordinates in the model: X = 8.5 m, Y = 5.5 m, Z = 0.8 m). The initial conditions and boundary conditions of the simulation were set as Tables 3 and 4 [41].
The convective heat transfer calculation was based on the standard model of FDS. The default radiation transport solver of FDS was enabled. The hall model boundaries were set to open. (The term “open” denotes a non-solid exterior boundary of the computational domain.) Gases were allowed to flow freely in and out. At the boundaries, the temperature and species mass fractions took on their respective exterior values if the flow was incoming, and their respective values in the grid cells adjacent to the boundaries if the flow was outgoing. It was a simple upwind boundary condition. The simulation calculation time was set to 600 s [42].
We set six monitoring points on the front altar table (No. 1 coordinates in the model: X = 8.5 m, Y = 5.5 m, Z = 1.1 m. The X and Y coordinates of the other five points remained unchanged. For each additional point, the Z coordinate increased by 0.5 m), 6 monitoring points on the doorway (No. 1 coordinates in the model: X = 7.68 m, Y = 0.0 m, Z = 1.1 m. The X and Y coordinates of the other five points remained unchanged. For each additional point, the Z coordinate increased by 0.5 m), and 2 slices (No. 1 coordinates above the altar table in the model: Y = 5.5 m; No. 2 coordinates above the doorway in the model: Y = 1.1 m) to monitor the heat release rate, smoke concentration, temperature, CO2 concentration, and visibility at different locations. Simultaneously, we also set monitoring points and slices at the exit of the Long’en Hall door to monitor the fire-spread.
4.3 Mesh Independence Verification
The mesh size of the model was selected as 0.25 × 0.25 × 0.25 m, and the number of mesh cells was 62,700. To verify the mesh independence, the four mesh sizes were set to 0.15 × 0.15 × 0.15 m, 0.25 × 0.25 × 0.25 m, 0.35 × 0.35 × 0.35 m, and 0.45 × 0.45 × 0.45 m. We set the test point to be on the altar table, 1.3 m above the ground, to measure the temperature values. Figure 6 shows that when the mesh size changed from 0.15 × 0.15 × 0.15 m to 0.25 × 0.25 × 0.25 m, the temperature of the test point changed a little with the increase of the number of cells. Refinement of the mesh (the addition of more cells) had a slight impact on the calculated results. It could be considered that the 0.25 × 0.25 × 0.25 m sized mesh reached mesh independence (Fig. 6).
5 Results and Discussion of Numerical Simulation
5.1 Heat Release Rate (HRR) and Fire Spread Process
The HRR is an important parameter that affects unit combustion of an object. It is also an important parameter to measure the degree of fire danger [43], and is usually used to indicate a fire’s progress over time. Table 5 shows the HRR data before and after treating wood with the flame retardant at different time points. As shown in Fig. 7, compared of heat release rate and the trend lines of yellow pine treated with flame retardants and without flame retardants, the HRR in Fig. 7 is at a stable value for the general trend. However sometimes, The HRR seems like a fluctuation because of the simulation method and flame oscillation. In the case of pristine wood, the fire expanded rapidly after ignition, and the HRR quickly increased as the material burned and released a large amount of heat, and the variation trend of the HRR with time was consistent with the fire spread trend. When the flame retardant was added to the wood, it burned more slowly and gave off less heat, about 50% lower than that of the pristine wood, indicating that the HRR was significantly low for the wood treated with the flame retardant and the fire was thus controlled.
Figure 8a–c shows the fire burning situations and corresponding flame heights at 100, 300, and 600 s before the wood was treated with the flame retardant, and Fig. 8d–f shows the fire burning situations and corresponding flame heights at 100, 300, and 600 s after the wood was treated with the flame retardant. Table 6 presents the flame heights before and after the wood was treated with the flame retardants at different points of time. According to Table 4, in the case of pristine wood, the difference in flame height ranged from 0 to 0.5 m higher than for wood treated with the flame retardant. The flame height decreased after some time as the fuel burned less.
5.2 Concentration and Spread of Smoke
The concentration and spread of smoke are essential parameters for evaluating the condition of smoke during combustion. If an object did not burn completely, it would produce a lot of smoke, obstruct people’s sight, prolong the evacuation and escape time at the fire scene, and increase the difficulty of extinguishing the fire. Smoke diffusion in buildings exhibit a unique fluidity, which is affected by the layout and connectivity of the interior space [37]. Therefore, the safety of smoke should not be greater than 5% for it to not significantly impact the safety of personnel evacuation. Figure 9a–c show the smoke concentrations before the wood was treated with the flame retardant, at 100, 300, and 600 s. Figure 9d–f show the smoke concentrations after the wood was treated with the flame retardant, at 100, 300, and 600 s. Tables 7 and 8 present the comparison of the smoke volume concentrations at different points of time (Test point above the table, 1.6 m above the ground) and (Test point above doorway, 1.6 m above the ground) before and after the wood was treated with the flame retardant.
Comparison of the smoke of yellow pine treated with and without flame retardants. (a–c) Spread of smoke of yellow pine without flame retardants after fire: (a) 100 s. (b) 300 s. c 600 s. (d–f) Spread of smoke of yellow pine treated with flame retardants after fire: (d) 100 s. (e) 300 s. f 600 s. (g) Smoke concentration (Test point above the table, 1.6 m above the ground) at different time points. (h) Smoke concentration (Test point above doorway, 1.6 m above the ground) at different time points
As shown in Fig. 9g, in the case of pristine wood, the smoke generated gradually after the fire, accumulated mainly at the top interface of the fire source space, and diffused to the adjacent opening space under the action of the air pressure difference. A large amount of smoke was generated about 4 s after the fire, and the corresponding smoke concentration reached its highest peak of 0.022 mol/mol. When the flame retardant was added to the wood, the smoke reduced significantly. The smoke concentration of the treated wood peaked at 0.0118 mol/mol about 7 s after the fire, 46.4% lower than that before treatment with the flame retardant (Test point above the table, 1.6 m above the ground). As shown in Fig. 9h (Test point above doorway, 1.6 m above the ground), in the case of pristine wood, the smoke concentration reached its highest peak of 1.71 E-04 mol/mol about 410 s after the fire. Afte r the flame retardant was added to the wood, the smoke concentration reached a peak value of 6.81E-05 mol/mol about 62 s after the fire, 60.2% lower than that before the wood was treated with the flame retardant, indicating that the addition of the flame retardant reduced both the concentration and emission of the smoke of ignited yellow pine.
5.3 Temperature of the Scene of the Fire
After the fire started, the temperature of the fire rose rapidly, and the burning rate was fast. If the open fire was not extinguished within a short period, a large fire would spread. We set the temperature slices above the table at 300 s to compare the temperatures of the fire scene under different working conditions (Fig. 10a and b). Six test points were set on each of the two slices, as shown in Fig. 5b. Tables 9 and 10 present the temperature data before and after the wood was treated with the flame retardant at different times.
(a) The temperature was high, and the fire flame was big in the case of yellow pine without flame retardants at 300 s after the fire. (b) When flame retardants were added into yellow pine, the flame shrank as the temperature dropped at 300 s after the fire. (c) Temperature (Test point above the table, 1.6 m above the ground) at different time points. (d) Temperature (Test point above doorway, 1.6 m above the ground) at different time points
Figure 10a shows that 300 s after the fire, the temperature was higher and the fire flame was bigger in the case of pristine yellow pine. As shown in Fig. 10b, when the flame retardant was added to the yellow pine, the flame shrank and the temperature dropped. As shown in Fig. 10c (Test point above the table, 1.6 m above the ground), in the case of pristine wood, about 61 s after the fire, the temperature reached its highest peak of about 804.5 °C. When the flame retardant was added to the wood, the temperature reached its highest peak of 609 °C about 242 s after the fire, 24.3% lower than that of the pristine wood. As shown in Fig. 10d (Test point above doorway, 1.6 m above the ground), in the case of pristine wood, about 411 s after the fire, the temperature reached a peak of 22.9 °C. After the flame retardant was added to the wood, the fire temperature reached a peak of 21.8 °C about 63 s after the fire, 4.8% lower than that of the pristine wood, indicating that the addition of flame retardant reduced the temperature at the fire-scene.
5.4 CO2 Concentration
Fires produce large amounts of CO2 (gas). Although CO2 is not toxic, upon accumulation in large amounts in a confined space, it can decrease oxygen concentration and cause suffocation. When CO2 levels reach 6–10%, people feel very uncomfortable and experience a shortness of breath, and when the levels exceed 10%, people lose consciousness within minutes, sustaining life-threatening injuries [44]. We set the CO2 concentration slices above the table at 300 s to compare the CO2 concentrations under different working conditions (Fig. 11a and b). Six test points were set on each of the two slices, as shown in Fig. 5b. Tables 11 and 12 present the comparison of the CO2 volume concentrations at different points of time (Test point above the table, 1.6 m above the ground and test point above doorway, 1.6 m above the ground) before and after the wood was treated with the flame retardants.
(a) There was a more comprehensive range of CO2 concentration, and the value of CO2 concentration was high in the case of yellow pine without flame retardants. (b) When flame retardants were added into yellow pine, the range of CO2 concentrations shrank, and the value of CO2 concentration dropped. (c) CO2 concentration (Test point above the table, 1.6 m above the ground) at different time points. (d) CO2 concentration (Test point above doorway, 1.6 m above the ground) at different time points
Figure 11a shows that about 300 s after the fire, there was a more comprehensive range of CO2 concentrations, and the CO2 concentration value was higher in the case of pristine yellow pine. As shown in Fig. 11b, when the flame retardant was added to the yellow pine, the range of CO2 concentrations shrank, and the CO2 concentration value dropped. As shown in Fig. 11c (Test point above the table, 1.6 m above the ground), in the case of pristine wood, the CO2 concentration reached its highest peak about 4 s after the fire, at 9.67E-02 mol/mol. When the flame retardant was added to the wood, the CO2 concentration about 8 s after the fire became 5.20E-02 mol/mol, 46.2% lower than that of the pristine wood. As shown in Fig. 11d (Test point above doorway, 1.6 m above the ground), in the case of pristine wood, about 80 s after the fire, the CO2 concentration reached a peak value of 0.001136 mol/mol. After the flame retardant was added to the wood, the fire CO2 concentration about 39 s after the fire reached a peak value of 0.000678 mol/mol, 40.3% lower than that of the pristine wood, indicating that the addition of the flame retardant reduced the concentration of the released CO2 upon the combustion of yellow pine.
5.5 Visibility
Solid particles in the smoke cause it to have a specific shade, which reduces the visibility of the fire, seriously affecting the evacuation and fire-fighting operations [45, 46]. Due to the settlement of smoke, when the fire inside a building develops to a particular stage, visibility at the building entrance reduces significantly [47]. Visibility is one of the most important factors affecting people’s escape after a fire. According to the provisions of “Code for fire protection design of buildings,” the visibility for a crowd unfamiliar with a building should be at least 13 m, while the visibility for the crowd familiar with the building should be at least 5 m. So, the critical hazard visibility is 5 m [48]. We set visibility slices above the doorway of Long’en Hall at 300 s to compare the visibilities under different working conditions (Fig. 12a–b). Six test points were set on each of the two slices, as shown in Fig. 5b. Tables 13 and 14 present the visibility data at different times before and after the wood was treated with the flame retardants.
(a) There was less visibility at the entrance of Long’en Hall in case of yellow pine without flame retardants. (b) When flame retardants were added into yellow pine, the range of visibility at the entrance of Long’en Hall expanded. (c) Visibility (Test point above the table, 1.6 m above the ground) at different time points. (d) Visibility (Test point above doorway, 1.6 m above the ground) at different time points
Figure 12a shows that about 300 s after the fire, there was less visibility at the entrance of Long’en Hall in the case of pristine yellow pine. As shown in Fig. 12b, when the flame retardant was added to the yellow pine, the range of visibility at the entrance of Long’en Hall extended. As shown in Fig. 12c (Test point above the table, 1.6 m above the ground), in the case of pristine wood, after the fire, the visibility reached a peak value of 0.12 m at 86 s. When the flame retardant was added to the wood, the visibility peaked at 0.16 m about 70 s after the fire, 33.3% lower than that of the pristine wood. As shown in Fig. 12d (Test point above doorway, 1.6 m above the ground), in the case of pristine wood, about 39 s after the fire, the visibility reached a peak value of 11.3 m. After the flame retardant was added to the wood, the fire visibility reached a peak value of 4.53 m about 80 s after the fire, 59.9% lower than that of the pristine wood, indicating that the addition of the flame retardant increased the visibility at the fire scene.
6 Conclusions
In this study, an experiment on the wood collected from the Long’en Hall of Fuling Mausoleum was treated with a flame retardant, and its corresponding numerical fire simulation by the FDS software was performed. Based on the analysis of the experimental and simulation results, the following conclusions were drawn:
-
(1) The flame retardant effect of the yellow pine soaked in a nitrogen and phosphorus flame retardant was pronounced. When the woodblock treated with flame retardant in the combustion process had been smouldering and it did not form an open flame, indicating an evident effect of the flame retardant on yellow pine. The flame retardant increased the ignition temperature of the wood, slowed down its combustion rate, and effectively delayed the combustion process by altering the pyrolysis method of the wood fibre to improve its combustion performance and fire resistance.
-
(2) Most areas of the woodblock of pristine yellow pine had been burned away, and the wood material had been charred. The depth of burning erosion ran through the whole block, which splitted into two parts. The burning erosion area of the woodblock treated with flame retardant was only part of the front side of it. The depth of burning erosion was only half as thick as the woodblock. The amount of CO gas produced upon the combustion of the yellow pine soaked in the flame retardant reduced by 24.9%, compared to that produced upon the combustion of pristine yellow pine. Similarly, the percentage of CO2 (gas) produced upon combustion of the treated yellow pine also decreased by 9.6%.
-
(3) In the numerical simulation, the variation trend of the HRR of the fire site over time was consistent with the movement of fire spread after ignition. Compared to the pristine wood, the flame retardant-treated wood exhibited a 53.1% lower HRR, indicating that the HRR reduced significantly upon treating the wood with the flame retardant and the fire was thus controlled. At the point above the fire (Test point above the table, 1.6 m above the ground), the addition of flame retardant to the yellow pine also reduced 46.4% concentration of smoke, and 46.2% concentration of CO2 (gas) upon its combustion, reduced 24.3% degrees of temperature of the fire site, and enhanced 33.3% of the visibility.
Data Availability
The data is available within the article.
References
Li C, Wang M (2009) Chinese ancient architecture and fire. Shanghai Scientific & Technical Publishers, Shanghai
Guo Z (2015) Disscussion on fire-fighting safety countermeasures of ancient architecture. Shanxi Architecture 41:250–251
Vijay PV, Gadde KT (2021) Evaluation of old and historic buildings subjected to fire. J Archit Eng. https://doi.org/10.1061/(ASCE)AE.1943-5568.0000456
Li L (2021) Simulation analysis of applicability of traditional fire prevention measures for ancient buildings. Fire Sci Technol 40:1332–1337
Hubbard C, Salem O (2022) Fire resistance of a fully concealed, moment-resisting new timber connection utilizing mechanically-fastened steel rods. Fire Saf J 129:103546
Hugi E, Weber R (2012) Fire behaviour of tropical and European wood and fire resistance of fire doors made of this wood. Fire Technol 48:679–698
Wang K (2017) Current situation and Countermeasures of fire safety of cultural relics and ancient buildings. Fire Protection Industry (electronic edition). 101–103.
Östman B, Boström L (2015) Fire protection ability of wood coverings. Fire Technol 51:1475–1493
Östman B, Tsantaridis L (2015) Fire scenarios for multi-storey façades with emphasis on full-scale testing of wooden façades. Fire Technol 51:1495–1510
Samanta P, Samanta A, Montanari C, Li Y, Maddalena L, Carosio F et al (2022) Fire-retardant and transparent wood biocomposite based on commercial thermoset. Compos A Appl Sci Manuf 156:106863
Yu PW (2021) Water damage of ancient wooden structures in high-pressure water mist fire suppression. Fire Science and Technology 40:668–671
Zhang M (2019) Fire prevention and control measures of museums and cultural relics and ancient buildings. Fire Sci Technol 38:713–715
Zhao Y (2018) Research on fire risk assessment method of ancient buildings. Fire Technol Product Inform 31:21–23
Carosio F, Cuttica F, Medina L, Berglund LA (2016) Clay nanopaper as multifunctional brick and mortar fire protection coating—wood case study. Mater Des 93:357–363
Hidalgo JP, Cowlard A, Abecassis-Empis C, Maluk C, Majdalani AH, Kahrmann S et al (2017) An experimental study of full-scale open floor plan enclosure fires. Fire Saf J 89:22–40
Stark NM, White RH, Mueller SA, Osswald TA (2010) Evaluation of various fire retardants for use in wood flour–polyethylene composites. Polym Degrad Stab 95:1903–1910
Yan L, Xu Z, Liu D (2019) Synthesis and application of novel magnesium phosphate ester flame retardants for transparent intumescent fire-retardant coatings applied on wood substrates. Prog Org Coat 129:327–337
Yan L, Xu Z, Deng N (2019) Effects of polyethylene glycol borate on the flame retardancy and smoke suppression properties of transparent fire-retardant coatings applied on wood substrates. Prog Org Coat 135:123–134
Wang T, Liu T, Ma T, Li L, Wang Q, Guo C (2018) Study on degradation of phosphorus and nitrogen composite UVcured flame retardant coating on wood surface. Prog Org Coat 124:240–248
Ma T, Li L, Wang Q, Guo C (2019) Construction of intumescent flame retardant and hydrophobic coating on wood substrates based on thiol-ene click chemistry without photoinitiators. Compos Part B-Eng 177:107357
Huang Y, Ma T, Wang Q, Guo C (2019) Synthesis of biobased flame-retardant carboxylic acid curing agent and application in wood surface coating. ACS Sustain Chem Eng 7:14727–14738
He X, Li X, Zhong Z, Mou Q, Yan Y, Chen H et al (2015) Effectiveness of impregnation of ammonium polyphosphate fire retardant in poplar wood using microwave heating. Fire Mater 40:818–825
Xu R, Xie Y, Tian J, Chen L (2021) Adsorbable organic halogens in contaminated water environment: a review of sources and removal technologies. J Clean Prod 283:124645
Morris AD, Muir DCG, Solomon KR, Teixeira CF, Duric MD, Wang X (2018) Bioaccumulation of polybrominated diphenyl ethers and alternative halogenated flame retardants in a vegetation–caribou–wolf food chain of the Canadian arctic. Environ Sci Technol 52:3136–3145
Wang X, Liu P, Xu G (2021) Influence of grass lawns on the summer thermal environment and microclimate of heritage sites: a case study of Fuling Mausoleum. China Heritage Sci 9:1–16
Wang X, Meng J, Zhu T, Zhang J (2019) Prediction of wind erosion over a heritage site: a case study of Yongling Mausoleum. China Built Heritage 3:41–57
Wang Y (2004) Fuling Mausoleum in Shenyang (Qing culture series). Shenyang Publishing House, Shenyang
Tencent website (2018) Ming building in Fuling mausoleum of Shenyang was destroyed by fire. https://new.qq.com/omn/20220507/20220507A0CKT300.html
Building Officials and Code Administrators International Inc. (1996) BOCA National Building Code.
Naval Engineering Standards (2013) NES713 determination of the toxicity index of the products of combustion from small specimens of materials. UK Ministry of Defence, London
Standardization Administration of China (2007) GB/T 8627 test method for density of smoke from the burning or decomposition of building materials. Standards Press of China, Beijing
McGrattan K, Hostikka S, McDermott R, Floyd J, Weinschenk CKO (2018) Fire dynamics simulator, technical reference guide, volume 3: validation, 6th ed.; Gaithersburg: National Institute of Standards and Technology, and VTT Technical Research Centre of Finland, Espoo, Finland.
Weinschenk CG, Overholt KJ, Madrzykowski D (2016) Simulation of an attic fire in a wood frame residential structure, Chicago, IL. Fire Technol 52:1629–1658
Zhang F, Shi L, Liu S, Shi J, Zhang J (2022) CFD-based framework for fire risk assessment of contiguous wood-frame villages in the western Hunan region. J Build Eng 54:104607
Cai N, Chow W-k (2014) Numerical studies on heat release rate in a room fire burning wood and liquid fuel. Building Simulation. 7:511–524
Kevin M et al (2007) Fire dynamics simulator (version 5) technical reference guide. Government Printing Office, Washington
Hui C, Xiao Z (2016) Risk analysis of fire spreading in traditional village dwellings. Build Sci 32:125–130
McGrattan K, Hostikka S, McDermott R, Floyd J, Vanella M (2018) Fire dynamics simulator technical reference guide volume 1: mathematical model. National Institute of Standards and Technology
Hongtaiji (2020) Introduction of flame retardant mechanism of phosphorus-nitrogen flame retardants and expansion flame retardants. http://www.szhongtaiji.com/newsdetail-17482562.html
Architectural Design Data Set Editorial Board (1994) Architectural design data collection, 2nd edn. China Architecture Press, Beijing
McGrattan K, Hostikka S, McDermott R, Floyd J, Weinschenk CKO (2018) Fire Dynamics Simulator User’s Guide, 6th ed. Gaithersburg: National Institute of Standards and Technology, and VTT Technical Research Centre of Finland, Espoo, Finland.
Lv S, Yang K (2014) PyroSim and its application- Pyrosim + pathfinder Chinese language learning and engineering application. Chemical Industry Press, Beijing
Zhang L, Han Z, Xie Q (2008) Full-scale experimental study on fire characteristics of typical upholstered furniture under different flow water spraying. Fire Sci 17:118–124
Zhao Y (2017) Research on smoke propagation and control measures of multi-storey building fire. Capital University of Economics and Business, Beijing
Suzuki S, Manzello SL (2021) Towards understanding the effect of cedar roof covering application on firebrand production in large outdoor fires. J Clean Prod 278:123243
Wills RF (2016) Development of a cyber physical system for fire safety. Springer International Publishing
Wang W, Wen H, Jia Y-F (2020) High-rise residential building fire simulation based on FDS. J Xi’an Univ Sci Technol 40:314–20
Ministry of Public Security of the People’s Republic of China (2018) Code for fire protection design of buildings: GB 50016–2014 (2018 version). China Planning Press, Beijing
Acknowledgements
The authors are also grateful to the students of Class 1302 in architecture, Shenyang University of Technology, for the surveying and mapping drawings of the Fuling Mausoleum.
Funding
This work was supported by the National Natural Science Foundation of China (Project No: 51978417), China Scholarship Council (File No.202108210150).
Author information
Authors and Affiliations
Contributions
XY-W has compiled the papers and produced the final manuscript, J-W, JH-W and GH-S have participated in the research. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare that they have no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, X., Wang, J., Wang, J. et al. Experimental and Numerical Simulation Analyses of Flame Spread Behaviour over Wood Treated with Flame Retardant in Ancient Buildings of Fuling Mausoleum, China. Fire Technol (2022). https://doi.org/10.1007/s10694-022-01311-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10694-022-01311-5