Abstract
The gynogenetic livebearing Amazon molly (Poecilia formosa) is a sexual parasite that exploits males of closely related species for sperm. This is needed as physiological stimulus for embryo development; however, none of the male’s genes are normally incorporated into the genome of the gynogenetic offspring. Mostly diploid individuals were reported from the natural habitats in North-Eastern Mexico and South-Eastern Texas but stable populations of triploids have been reported from the Río Soto la Marina drainage and in the Río Guayalejo in North-Eastern Mexico. Triploidy is the result of defects in the mechanisms that normally clear the host sperm from the ameiotic diploid egg. Triploids also reproduce gynogenetically and their frequencies fluctuate markedly between years, seasons, and localities. To understand the dynamics of this mating system, it is important to understand the relative reproductive success of triploids and diploids. We hypothesize that triploids should have a selective advantage over diploids due to heterosis and/or gene redundancy based on the additional genetic material from the sexual host. However, clonal competition experiments revealed a clear reproductive advantage of diploids competing with triploids. This result contradicts not only our hypothesis but also the stable co-existence of diploids and triploids in natural habitats. Frequency dependent selection, niche partitioning and environmental heterogeneity are discussed as possible explanations.
Similar content being viewed by others
Introduction
The advantages and disadvantages of unisexual reproduction are still a matter of debate (West et al. 1999). While unisexually reproducing species should have the clear advantage of not having to produce males, and therefore should have a faster reproductive rate than sexually reproducing species they should also be slower to evolve and evolutionary short lived due to an accumulation of deleterious mutations (Kondrashov 1988; Bell 1982; Muller 1932). In asexuals no genotype can ever produce offspring with fewer mutations than its own load. Therefore, the accumulation of deleterious mutations will lead to a fitness decline and should in theory lead to the extinction of parthenogenetic forms within 104–105 generations (Lynch et al. 1993; Lynch and Gabriel 1990). Strategies to overcome this constraint include forms of ‘rare sex’ and have been hypothesized to be cyclical parthenogenesis (Kondrashov 1984), occurrence of rare males (Martens 1998), simultaneous occurrence of sexual and asexual (mixed) reproduction (Schön et al. 2000; Martens 1998), and paternal leakage in species reproducing by sperm-dependent parthenogenesis, also called gynogenesis or kleptospermy (Bogart et al. 2007; Schartl et al. 1995a). In unisexual vertebrates cyclic changes of reproductive mode, hermaphrodites, and mixed reproduction are unheard of. To overcome the restraints of unisexual reproduction gynogenetic all-female vertebrates therefore may rely on paternal introgression, eventually leading to ploidy elevation (Beukeboom 2007; D’Souza et al. 2006). Polyploidy is the heritable condition of possessing more than two complete sets of chromosomes. Polyploids are very common among plants, but can also be found among fish and amphibians. A specific link between polyploidy and asexual reproduction has been observed in insects, flatworms, fishes, and reptiles (Mogie 2007; Mable 2004; Otto and Whitton 2000; Benazzi and Lentati 1999; Lynch et al. 1993; Avise et al. 1992; Suomalainen et al. 1987; Benazzi Lentati 1979; Schultz 1969; Christensen 1960). For unisexual species there are some advantages as well as disadvantages of becoming polyploid (for an overview see Comai 2005 and Otto 2007). Disadvantages stated for polyploidy are so far mainly based on theoretical considerations and include the disrupting effects of nuclear and cell enlargement, an increased probability of polyploid mitosis and meiosis to produce aneuploid cells, as well as unbalanced gene expression (Comai 2005; Pandian and Koteeswaran 1998; Horner and Macgregor 1983). It is known that higher DNA contents are correlated with bigger cell sizes. As cell volume increases, however, the ratio of total surface area must decrease which is expected to change the functional ability of polyploids (Kozlowski et al. 2003; Oliva-Teles and Kaushik 1987). On the other hand, cell number in polyploid vertebrates is reduced to maintain the normal size of the individual. Some studies show a decrease in the number of erythrocytes per unit volume of blood in triploid fishes (reviewed by Pandian and Koteeswaran 1998). Horner and Macgregor (1983) found a positive relationship of genome size and cell cycle time: The larger the genome the larger the cell and the longer the cell cycle time. Prolonged cell cycle times might negatively influence development and generation time as well as gamete production and wound healing.
Proposed advantages of polyploidy are heterosis and gene redundancy (Comai 2005). Experimental evidence has been provided that allopolyploids (i.e., polyploids with chromosomes derived from different species, e.g., by hybridisation) are more vigorous than their diploid progenitors (heterosis). This might be due to higher immunological resistance (Pandian and Koteeswaran 1998), higher survival and faster growth (Pandian and Koteeswaran 1998), sexual mimicry (Beukeboom and Vrijenhoek 1998), local adaptation (Beukeboom and Vrijenhoek 1998), or general higher within individual genetic variability (Rasch and Balsano 1989). Gene redundancy will shield polyploids from the deleterious effect of mutations (Otto and Whitton 2000). Moreover, the increase in gene copy number resulting from polyploidisation may lead to increased functional gene diversity. It has been found in bdelloid rotifers that additional gene copies have diverged in function so that the proteins they encode play complementary roles in survival of dry conditions (Pouchkina-Stantcheva et al. 2007). This “neo”- or “sub”-functionalisation of gene copies increases the functional gene diversity and therefore the ecological adaptation of species (Pouchkina-Stantcheva et al. 2007; Taylor and Raes 2004; Otto and Whitton 2000).
A well-studied vertebrate example for a unisexual species showing different ploidy levels is the Amazon molly (Poecilia formosa). It arose by hybridisation between two closely related species, P. mexicana and P. latipinna. Molecular analyses date its hybrid origin to approximately 280.000 years/840,000 generations ago (Lampert and Schartl 2008; Schartl et al. 1995b; Avise et al. 1991). This contradicts the predictions on the longevity of ameiotic species of 104–105 generations (Loewe and Lamatsch 2008; Judson and Normark 1996; Lynch and Gabriel 1990; Muller 1964).
Poecilia formosa is a small live-bearing fish which inhabits fresh water streams of Northern Mexico and Southern Texas. Its mode of reproduction is gynogenesis, i.e., embryogenesis is triggered by sperm of males of closely related species, mostly P. mexicana and P. latipinna, without fertilizing the egg. The mechanism which normally excludes the sperm from the oocyte may fail resulting in actual syngamy of the unreduced diploid egg nucleus (‘ml’ according to its hybrid origin) and the sperm nucleus (‘m’ or ‘l’). This will lead to triploid offspring (‘mlm’ or ‘mll’). Only a few triploid hybrids have been identified where P. formosa is sympatric with P. latipinna (four specimens ‘mll’ from the Rio Grande) or with P. mexicana mexicana (one specimen ‘mlmm’ from the Rio Tuxpan) (Monaco et al. 1984). The majority of triploid unisexuals occur where P. formosa is sympatric with P. mexicana limantouri (‘mlml’). In natural habitats mostly diploids occur, however, stable triploid populations co-occur with diploids in mixed groups in the Río Soto la Marina drainage (Lampert et al. 2005; Rasch and Balsano 1974) and in the Río Guayalejo (Schories et al. 2007). These have been found to be of the ‘lmml’ type according to the presence of P. m. limantouri as host. The overall frequency of triploid females ranges from 5% to 20% (Lampert et al. 2005; Rasch and Balsano 1974), however, significant fluctuations between years, season and localities have been reported with up to 80% triploids on local scale (Rasch and Balsano 1974). Despite a slightly—but not significantly lower reproductive output of triploid females (Balsano et al. 1985; Rasch and Balsano 1974) natural selection seems to favor triploid juveniles over diploid ones (Balsano et al. 1983; Rasch and Balsano 1974).
Based on the latter observation and the theoretically positive effect of the increased heterosis in triploids due to the additional genetic material from the sexual host, we hypothesized that triploids should have a selective advantage over diploids. We tested this hypothesis in competition experiments where under identical environmental conditions triploids competed with diploids for access to males. Unexpectedly, we found that diploids outcompeted triploids in these experiments.
Materials and methods
Experimental set-up
An equal number of diploid and triploid P. formosa of laboratory bred strains from the same locality (Barretál, Río Purificación, Tamaulipas, Mexico) were put together in one tank with an approximate volumes of 360–576 l (Table 1). Prior to the experiment ploidy has been confirmed by flow cytometry (Lamatsch et al. 2000). To simplify the experimental set up and interpretation, a single diploid and a single triploid clone were used in the experiments (Lampert et al. 2008). This reflects the natural situation for the triploids as triploids show a very low clonal variability in the field (Lampert et al. 2005), and the clone used in the experiments is the dominant clone in the field. For the diploids, clonal frequencies changed between the years but the clone used for our experiment was commonly found in field sites (KPL unpublished). Care was taken that diploid and triploid females used in each experiment did not differ significantly in size (standard length, recorded before start) (Table 1). Since no significant differences between birth size among diploid and triploid P. formosa females could be detected under lab conditions (Lamatsch 2001) we assumed equal size reflects equal age under the same laboratory conditions. Varying starting numbers of individuals (14–20) were due to varying availability of equally sized individuals of both ploidy levels. All P. formosa were virgins, and two males of P. mexicana limantouri (Barretál, Rio Purificación, Tamaulipas, Mexico) were added as sperm donors after 1 week of acclimation resulting in a sex ratio of 1:7–1:10 (males/females). Due to the presence of diploid and triploid asexual females in addition to females of the sexual host species the sex ratio in natural habitats is extremely female-biased. In 1998 we found the female sex ratio to be 4.6 and 7.4 times the number of males (Döbler 1998) which is in accordance with data from Rasch and Balsano (1974) who found sex ratios from 5.9 to 6.7. We used an sex ratio of 7 to 10 times considering that access to female under aquarium conditions might be higher as the fish are not concerned with predator avoidance, finding food or seeking for mating partners in larger water bodies. Since most receptive females in natural habitats were inseminated (DKL pers. observation), these extremely female-biased sex ratios might at least partly be counterbalanced by the high potential rate of reproduction of males and the asynchrony in female receptivity.
The presence of males was controlled at least weekly and males were substituted if necessary (approximately every 6–8 weeks). Dead fish were stored in 70% ethanol for ploidy measurement to control for varying mortality rates in diploids and triploids throughout the course of the experiment.
In Würzburg this experiment was set up six times independently (designated as ‘W’ trials). In addition, two more experimental trials were set up in Seewiesen (designated as ‘S’ trials). To control for potential spontaneous changes in ploidy level (triploids loosing a set of chromosomes and/or diploids gaining one), two tanks were installed containing only the diploid or the triploid clone, respectively (N = 20 females, one male), and randomly checked for ploidy approximately every 6 months. All fish were maintained for 18 months under standard conditions as described for Xiphophorus (Kallman 1975) at 24–27°C with a 13 h light/11 h dark light cycle and fed twice a day ad libitum.
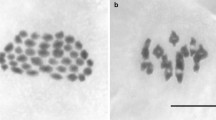
After 12 and 18 months the ploidy level of all fish was measured by using the dorsal fin for flow cytometric measurement as described in Lamatsch et al. (2000). The DNA content of the cells was determined using chicken red blood cells as a reference (Vinogradov 1998).
Statistics
To test for significant deviations from the initial proportion of diploid and triploid individuals in the trials χ 2-goodness-of-fit tests were performed with the data from 12 and 18 months. All P-values were calculated for a two-tailed probability of deviation from the initial status of the experiments (50% diploids, 50% triploids). Bonferroni correction was applied for multiple testing but did not change the outcome of the results as those were highly significant. To test for an overall trend in the experimental trials a Fisher exact test was performed for both data points (12 and 18 months) comparing the number of trials where diploids out-competed the triploids against the assumed outcome postulating a triploid advantage.
Results and discussion
Occasional leakage of genes from a paternal host into a sperm-dependent clone may provide a source of adaptive variation and possibly an avenue for the avoidance of ‘Muller’s ratchet’ (Muller 1964) or ‘mutational meltdown’ (Lynch et al. 1993) by adding freshly recombined genetic material (Bogart et al. 2007; Schartl et al. 1995a; Wright and Lowe 1967). This mechanism might allow compensation for malfunctioning genes. Although heterosis may be involved in the success of asexual polyploids, clear documentation of their superior fitness to parental precursors is difficult to obtain since it requires that all biotypes be measured under the same conditions (Otto and Whitton 2000; Schultz and Fielding 1989). To study possible fitness differences between triploid and diploid clones of the Amazon molly, clonal competition experiments were set up with equal numbers of diploid and triploid virgin P. formosa using only two P. m. limantouri males as sperm donors in each replicate representing the natural sex ratios.
Fish gave birth approximately from 2 months on after adding the P. mexicana males to the virgin females which ranges within the reproductive rate of animals bred in the laboratory. Reproductive rates varied greatly between the experimental trials: While in all W-trials fish numbers increased noticeably, the fish in the S-trials did not reproduce at the same rate (Table 2). Since this was most likely due to locally different (water) conditions the S-trials were analysed separately.
After 12 months three of the six W-experiments showed a significant bias towards diploid individuals (Table 2), two did not differ significantly from a 50% diploid 50% triploid distribution (W3, W5) and one experiment (W4) showed a slightly significant triploid dominance. After 18 months five out of six experiments showed a highly significant diploid bias (Table 2), while W4 showed a balanced proportion of diploids and triploids. A Fisher exact test of these results compared to the expected triploid dominance clearly showed a significant bias towards diploid dominance after 12 (P = 0.033) and 18 months (P = 0.002).
Therefore, the individual trials as well as an overall analysis of the clonal competition experiments revealed a clear reproductive advantage of diploids competing with triploids. This result was supported by the S-experiments (Table 2). The rather unexpected outcome of triploids being inferior to diploids may be explained by several mechanisms:
-
(1)
Disproportional amounts of death rates in triploids, in particular during the early phases of the experiments could have led to an early diploid bias in the experiments explaining the clear dominance of diploids at the end of the experiments. Flow cytometric measurements of deceased fishes, however, showed that the higher fitness of diploids is not based on higher mortality of triploids, since their overall mortality rates were not significantly different (χ2 = 0.071; P = 0.79; Supplementary Fig. 1): in diploids 28.65% (302/1,054) died, in triploids 27.51% (166/229). Even though it looks like diploids have a higher mortality in experiments 5 and 6, diploids were also much more common than triploids in the tanks at these stages of the experiment, and therefore relative death rates did not differ between ploidy levels.
-
(2)
A spontaneous change of ploidy. Earlier observations from the laboratory had shown that triploidisation happens in laboratory breeding strains of P. formosa (Nanda et al. 1995). It does, however, occur at low frequencies and the resulting females are sterile. A random combination of genotypes is obviously not sufficient to produce fertile new clones. According to recent analyses, the origin of natural triploids in P. formosa was restricted to only a few—successful—introgression events (Lampert et al. 2005; Schories et al. 2007). No change of ploidy was observed in the controls (N = 141) during the entire course of the experiment. Therefore, spontaneous ploidy elevations are negligible for our experiments. The loss of a chromosome set has never been observed under laboratory conditions and can therefore be excluded as a main cause of diploid dominance.
-
(3)
The most likely explanation for our experimental results is that under standardized lab condition i.e., in a physically stable, predator-free environment with food readily available and sole competition for mates triploids are inferior competitors to diploids. Under natural conditions, however, triploids make up a fluctuating but stable part of the population, and are found in mixed schools with diploids. Therefore, we have to assume that there are environmental conditions that stabilize this co-existence. Different environmental conditions e.g., might explain the initial dominance of triploids in W4.
Several possibilities exist to explain the occurrence of mixed ploidy levels within populations due to balancing selection: frequency dependent selection might favor triploids if the frequency of diploids becomes too high and vice versa. This might explain the fluctuating frequencies of triploids between different years (Rasch and Balsano 1974).
Another mechanism by which natural selection can preserve two or more phenotypic forms is heterozygote advantage. Polyploids might be less susceptible to parasites due to the presumed higher individual genetic variability (Rasch and Balsano 1989). Since triploids in P. formosa, however, show an unexpectedly low genotypic diversity (Lampert et al. 2005), they might actually be more exposed to parasitism by representing the most common genotype (Lively et al. 1990). Tobler and Schlupp (2005) could not detect differences in parasite load between asexual P. formosa and its sexual host P. latipinna, however, only diploid asexuals have been investigated. Whether the significantly higher genotypic diversity of diploid P. formosa versus triploid P. formosa is reflected in parasite load has to be investigated. Fish mycobacteriosis is a chronic bacterial disease that has the potential for infecting most fish species from both freshwater and saltwater habitats. Its pathogen, Mycobacterium marinum is known as a constitutive laboratory (Ranzani-Paiva et al. 2004) and natural (Poort et al. 2006; Lansdell et al. 1993) parasite, and seems a good target to address the above raised question.
A third explanation could be environmental heterogeneity. Triploids may be better suited to withstand stressful conditions due to physiological differences, e.g., in metabolic rates, resulting from the cell enlargements (Kozlowski et al. 2003; Oliva-Teles and Kaushik 1987). During late spring months in North-Eastern Mexico the rivers slowly dry up and eventually small pools detached from the stream will form showing high population densities, high temperatures and low oxygen levels. High frequencies of triploids reported from these rest water pools (Lamatsch 2001; Balsano et al. 1981) and higher numbers of triploids in summer months (Balsano and Rasch 1974) may indicate a higher tolerance towards stressful environmental conditions. Differences in the metabolic rate between diploid and triploid P. formosa were not tested in this experimental set-up but need to be investigated to evaluate whether this hypothesis is a reasonable explanation.
Another possibility for co-existence would also be niche partitioning as found in Poeciliopsis (Vrijenhoek 1978). Niche partitioning in P. formosa, as well as in their sexual hosts (P. mexicana, P. latipinna) has been suggested by Balsano et al. (1981) who found sexual females to be more abundant in headwater localities whereas unisexuals increased downstream. Their data support the hypothesis of microhabitat segregation among the three types of females (P. mexicana, diploid and triploid P. formosa) although both unisexual and sexual females were usually found in a given habitat. This hypothesis is currently being tested by us (Lamatsch and Schlupp et al. in preparation). Weiss et al. (1999) proposed that a combination of advantages and disadvantages of both biotypes account for a stable coexistence of different ploidy levels. Despite a large number of possible hypotheses explaining the pattern found here, the question whether intraspecific polyploidy is a form of genetic variation that may expand environmental tolerance requires more theoretical and experimental analyses.
References
Avise JC, Trexler JC, Travis J, Nelson WS (1991) Poecilia mexicana is the recent female parent of the unisexual fish P. formosa. Evolution 45:1530–1533
Avise JC, Quattro JM, Vrijenhoek RC (1992) Molecular clones within organismal clones. Evol Biol (26), 225–245. Plenum Press, New York
Balsano JS, Rasch EM (1974) Biochemical and cytogenetic studies of Poecilia from eastern mexico. I. Comparative microelectrophoresis of plasma proteins of seven species. Rev Biol Trop 21:229–257
Balsano JS, Kucharski K, Randle EJ, Rasch EM, Monaco PJ (1981) Reduction of competition between bisexual and unisexual females of Poecilia in north-eastern Mexico. Env Biol Fish 6:39–48
Balsano JS, Rasch EM, Monaco PJ (1983) Reproductive interactions within bisexual/unisexual complexes of Poecilia from the Rio Purificación in north-eastern Mexico. Nat Georgr Soc Res Repts 1976 Projekts:113–135
Balsano JS, Randle EJ, Rasch EM, Monaco PJ (1985) Reproductive behavior and the maintenance of all-female Poecilia. Env Biol Fish 12:251–263
Bell G (1982) The masterpiece of nature: the evolution of sex and the genetics of sexuality. University of California Press, Berkeley
Benazzi Lentati G (1979) Polyploidy and types of development in the triclad Dugesia lugubris S.L.: interaction between the ploidy level and the origin of amphimixis or pseudogamy. Caryologia 32:247–263
Benazzi M, Benazzi Lentati G (1999) Gynogenesis and polyploidy in the animal kingdom. Riv Biol 92:452–454
Beukeboom LW (2007) Sex to some degree. Heredity 98:123–124
Beukeboom LW, Vrijenhoek RC (1998) Evolutionary genetics and ecology of sperm-dependent parthenogenesis. J Evol Biol 11:755–782
Bogart JP, Bi K, Fu J, Noble DWA, Niemeitz A (2007) Unisexual salamanders (Genus Ambystoma) present a new reproductive mode for eurkaryotes. Genome 50:119–136
Christensen B (1960) A comparative cytological investigation of the reproductive cycle of an amphimictic diploid and a parthenogenetic triploid form of Lumbricillus lineatus (O.F.M.) (Oligochaeta, Enchytraeidae). Chromosoma 11:365–379
Comai L (2005) The advantages and disadvantages of being polyploid. Nat Rev Genet 6:836–846
Döbler M (1998) Zum Fortpflanzungsmodeus des Amazonenkärpflings [Poecilia formosa (Girard 1859)]. Thesis/Dissertation, Zoologisches Institut und Zoologisches Museum der Universität Hamburg
D’Souza TG, Schulte RD, Schulenburg H, Michiels NK (2006) Paternal inheritance in parthenogenetic forms of the planarian Schmidtea polychroa. Heredity 97:97–101
Horner HA, Macgregor HCC (1983) Value and cell volume: their significance in the evolution and development of amphibians. J Cell Sci 63:135–146
Judson OP, Normark BN (1996) Ancient asexual scandals. Trends Ecol Evol 11:41–46
Kallman KD (1975) The platyfish, Xiphophorus maculatus. In: King RC (ed) Handbook of genetics. Plenum Publishing Corp, New York, pp 81–132
Kondrashov AS (1984) A possible explanation of cyclical parthenogenesis. Heredity 52:307–308
Kondrashov AS (1988) Deleterious mutations and the evolution of sexual reproduction. Nature 336:435–440
Kozlowski J, Konarzewski M, Gawelczyk AT (2003) Cell size as a link between noncoding DNA and metabolic rate scaling. Proc Natl Acad Sci USA 100:14080–14085
Lamatsch DK (2001) Molecular and cytogenetic investigations on paternal leakage in the sperm-dependent parthenogen, Poecilia formosa. Thesis/Dissertation, University of Wuerzburg
Lamatsch DK, Geiger M, Schlupp I (in preparation) Investigations on the habitat choice of diploid and triploid P. formosa, and their hosts P. mexicana and P. latipinna
Lamatsch DK, Steinlein C, Schmid M, Schartl M (2000) Noninvasive determination of genome size and ploidy level in fishes by flow cytometry: detection of triploid Poecilia formosa. Cytometry 39:91–95
Lampert KP, Schartl M (2008) The origin and evolution of a unisexual hybrid: Poecilia formosa. Philos Trans R Soc Lond B, in press. doi:10.1098/rstb.2008.0040
Lampert KP, Lamatsch DK, Epplen JT, Schartl M (2005) Evidence for a monophyletic origin of triploid clones of the Amazon molly, Poecilia formosa. Evolution 59:881–889
Lampert KP, Lamatsch DK, Fischer P, Schartl M (2008) A tetraploid Amazon molly, Poecilia formosa. J Hered 99:223–226
Lansdell W, Dixon B, Smith N, Benjamin L (1993) Isolation of several Mycobacterium species from fish. J Aquat Anim Health 5:73–76
Lively CM, Craddock C, Vrijenhoek RC (1990) The Red Queen hypothesis supported by parasitism in sexual and clonal fish. Nature 344:864–866
Loewe L, Lamatsch DK (2008) Muller’s ratchet may threaten the Amazon molly and other ancient asexuals. BMC Evol Biol 8:88–108
Lynch M, Gabriel W (1990) Mutation load and the survival of small populations. Evolution 44:1725–1737
Lynch M, Burger R, Butcher D, Gabriel W (1993) The mutational meltdown in asexual populations. J Hered 84:339–344
Mable BK (2004) Why polyploidy is rarer in animals than in plants: myths and mechanisms. Biol J Linn Soc 82:453–466
Martens K (1998) Sex and ostracods: a new synthesis. In: Martens K (ed) Sex and parthenogenesis: evolutionary ecology of reproductive modes in non-marine Ostracods. Backhuys Publishers, Leiden, pp 295–322
Mogie M (2007) On the relationship between asexual reproduction and polyploidy. J Theor Biol 122:493–498
Monaco PJ, Rasch EM, Balsano JS (1984) Apomictic reproduction in the Amazon molly, Poecilia formosa, and its triploid hybrids. In: Turner BJ (ed) Evolutionary genetics of fishes. Plenum Press, New York, NY, USA, pp 311–328
Muller HJ (1932) Some genetic aspects of sex. Am Nat 66:118–138
Muller HJ (1964) The relation of recombination to mutational advance. Mutat Res 1:2–9
Nanda I, Schartl M, Feichtinger W, Schlupp I, Parzefall J, Schmid M (1995) Chromosomal evidence for laboratory synthesis of a triploid hybrid between the gynogenetic teleost Poecilia formosa and its host species. J Fish Biol 47:619–623
Oliva-Teles A, Kaushik SJ (1987) Metabolic utilization of diets by polyploid rainbow trout (Salmo gairdneri). Comp Biochem Physiol A 88:45–47
Otto SP (2007) The evolutionary consequences of polyploidy. Cell 131:452–462
Otto SP, Whitton J (2000) Polyploid incidence and evolution. Annu Rev Genet 34:401–437
Pandian TJ, Koteeswaran R (1998) Ploidy induction and sex control in fish. Hydrobiologia 384:167–243
Poort MJ, Whipps CM, Watral VG, Font WF, Kent ML (2006) Molecular characterization of a Mycobacterium species in non-native poeciliids in Hawaii using DNA sequences. J Fish Dis 29:181–185
Pouchkina-Stantcheva NN, McGee BM, Boschetti C, Tolleter D, Chakrabortee S, Popova AV, Meersman F, Macherel D, Hincha DK, Tunnacliffe A (2007) Functional divergence of former alleles in an ancient asexual invertebrate. Science 318:268–271
Ranzani-Paiva MJT, Ishikawa CM, Eiras ACd, Silveira VRd (2004) Effects of an experimental challenge with Mycobacterium marinum on the blood parameters of Nile tilapia, Oreochromis niloticus (Linnaeus, 1757). Braz Arch Biol Technol 47:945–953
Rasch EM, Balsano JS (1974) Biochemical and cytogenetic studies of Poecilia from eastern Mexico. II. Frequency, perpetuation, and probable origin of triploid genomes in females associated with Poecilia formosa. Rev Biol Trop 21:351–381
Rasch EM, Balsano JS (1989) Trihybrids related to the unisexual molly fish, Poecilia formosa. In: Dawley RM, Bogart JP (eds) Evolution and ecology of unisexual vertebrates. New York State Museum, Albany, New York, pp 252–267
Schartl M, Nanda I, Schlupp I, Wilde B, Epplen JT, Schmid M, Parzefall J (1995a) Incorporation of subgenomic amounts of DNA as compensation for mutational load in a gynogenetic fish. Nature 373:68–71
Schartl M, Wilde B, Schlupp I, Parzefall J (1995b) Evolutionary origin of a parthenoform, the Amazon molly Poecilia formosa, on the basis of a molecular genealogy. Evolution 49:827–835
Schön I, Gandolfi A, di Masso E, Rossi V, Griffith HI, Martens K, Butlin RK (2000) Persistence of asexuality through mixed reproduction in Eucypris virens (Crustacea, Ostracoda). Heredity 84:161–169
Schories S, Lampert KP, Lamatsch DK, Garcia de Leon FJ, Schartl M (2007) Analysis of a possible independent origin of triploid P. formosa outside of the Río Purificación river system. Front Zool 4:13
Schultz RJ (1969) Hybridization, unisexuality, and polyploidy in the teleost Poeciliopsis (Poeciliidae) and other vertebrates. Am Nat 103:605–619
Schultz RJ, Fielding E (1989) Fixed genotypes in variable environments. In: Dawley RM, Bogart JP (eds) Evolution and ecology of unisexual vertebrates. New York State Museum, Albany, New York, pp 32–38
Suomalainen E, Saura A, Lokki J (1987) Cytology and evolution in parthenogenesis. CRC Press, Boca Raton, Florida
Taylor JS, Raes J (2004) Duplication and divergence: the evolution of new genes and old ideas. Annu Rev Genet 38:615–643
Tobler M, Schlupp I (2005) Parasites in sexual and asexual molly species of the genus Poecilia (Poeciliidae, Teleostei): a case for the Red Queen? Biol Lett 1:166–168
Vinogradov AE (1998) Genome size and GC-percent in vertebrates as determined by flow cytometry: the triangular relationship. Cytometry 31:100–109
Vrijenhoek RC (1978) Coexistence of clones in a heterogeneous environment. Science 199:549–552
Weiss MM, Hermsen MA, Meijer GA, van Grieken NC, Baak JP, Kuipers EJ, van Diest PJ (1999) Comparative genomic hybridisation. Mol Pathol 52:243–251
West SA, Lively CM, Read AF (1999) A pluralistic approach to sex and recombination. J Evol Biol 12:1003–1012
Wright JW, Lowe CH (1967) Evolution of the alloploid parthenospecies Cnemidophorus tesselatus (Say). Mammal Chromosome Newslett 8:95–96
Acknowledgments
We thank G. Schneider, H. Schwind, and P. Weber for breeding the fish, and A. Chadt, J. Delfgaauw, M. Hidding, C. Ryser, and T. Schäfer for technical support. Financial support for this study was granted by the DFG (SFB 567 “Mechanismen der interspezifischen Interaktion von Organismen”), and Fonds der Chemischen Industrie. I.S. was supported by a Heisenberg Fellowship. We are grateful to the Max-Planck-Society for financial support to M. Geiger. We owe special thanks to W. Wickler for his great support of this project.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Supplemental Figure 1
Death of diploid (black columns) and triploid (hatched columns) fishes during 18 months of each Würzburg experiment (DOC 116 KB)
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Lamatsch, D.K., Lampert, K.P., Fischer, P. et al. Diploid Amazon mollies (Poecilia formosa) show a higher fitness than triploids in clonal competition experiments. Evol Ecol 23, 687–697 (2009). https://doi.org/10.1007/s10682-008-9264-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-008-9264-2