Abstract
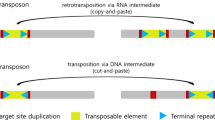
Transposable elements are presents in all known genomes so far, and have the faculty of changing their genomic location and/or number of copies within the genome. They are mobile endogenous genetic elements, with a large variety of structure and transposition mechanism. In crops, they compose the major part of the nucleic DNA, up to 80% in some cereals like maize and wheat. Despite their omnipresence, they are largely unknown and uncharacterized within the Poaceae family. In this review, we describe a possible classification of the elements present in this family, some of their known transposition mechanism, their known activity and possible action in crops, and their possible origin.
Similar content being viewed by others
References
Adé, J. & F.J. Belzile, 1999. Hairpin elements, the first family of foldback transposons (FTs) in Arabidopsis thaliana. Plant J 19(5): 591–597.
Ananiev, E.V., R.L. Phillips & H.W. Rines, 1998. Chromosome-specific molecular organization of maize (Zea mays L.) centromeric regions. Proc Natl Acad Sci USA 95: 13073–13078.
Arkhipova, I. & M. Meselson, 2000. Transposable elements in sexual and ancient asexual taxa. Proc Natl Acad Sci USA 97(26): 14473–14477.
Arnaud, Ph., Y. Yukawa, L. Lavie, T. Pélissier, M. Suguira & J.M. Deragon, 2001. Analysis of the SINE S1 Pol III promoter from Brassica; impact of methylation and influence of external sequences. Plant J 26(3): 295–305.
Avramova, Z., A., Tikhonov, Ph. SanMiguel, Y.-K., Jin, C., Liu, S.-S., Woo, R.A. Wing & J.L., Bennetzen, 1996. Gene identification in a complex chromosomal continuum by local genomic cross-referencing. Plant J 10(6): 1163–1168.
Bannister, J.V. & M.W., Parker, 1985. The presence of a copper/zinc superoxide dismutase in the bacterium Photobacterium leiognathi: A likely case of gene transfer from eucaryotes to prokaryotes. Proc Natl Acad Sci USA 82(1) 149–152.
Baulcombe, D.C., 2000. Unwiding RNA silencing. Science 290: 1108–1109.
Beall, R. & D.C. Rio, 1996. Drosophila IRBP/Ku p70 corresponds to the mutagen-sensitive mus309 gene and is involved in P-element excision in vivo. Genes Dev 10(8): 921–933.
Bender, J., 1998. Cytosine methylation of repeated sequences in eukaryotes: The role of DNA pairing. TIBS 23: 252–256.
Bennetzen, J.L., 2000. Transposable element contributions to plant gene and genome evolution. Plant Mol Biol 42: 251–269.
Bennetzen, J.L., 2002. Mechanism and rates of genome expansion and contraction in flowering plants. Genetica 115: 29–36.
Bhattacharyya, M.K., A.M. Smith, T.H. Ellis, C. Hedley & C. Martin, 1990. The wrinkled-seed character of pea described by Mendel is caused by a transposon-like insertion in a gene encoding starch-branching enzyme. Cell 60(1): 115–122.
Boeke, J.D., 1989. Transposable elements in Saccharomyces cerevisiae. In: D.E. Berg & M.H. Howe (Eds.): Mobile DNA, pp. 335–374, American Society for Microbiology, Washington, DC.
Boutabout, M., M. Wilhelm & F.-X. Wilhelm, 2001. DNA synthesis fidelity by the reverse transcriptase of the yeast retrotransposon Tyl. Nucleic Acids Res 29(11): 2217–2222.
Bowen, N.J. & J.F. McDonald, 2001. Drosophila euchromatic LTR retrotransposons are much younger than the host species in which they reside. Genome Res 11: 1527–1540.
Bureau, T.E. & S.R. Wessler, 1994a. Mobile inverted-repeat elements of the Tourist family are associated with the genes of many cereal grasses. Proc Natl Acad Sci USA 91: 1411–1415.
Bureau, T.E. & S.R. Wessler, 1994b. Stowaway: A new family of inverted repeat elements associated with the genes of both monocotyledonous and dicotyledonous plants. Plant Cell 6: 907–916.
Bureau, T.E., S.E. White & S.R. Wessler, 1994. Transduction of a cellular gene by a plant retroelement. Cell 77: 479–80
Capy, P., 2003 (Eds.). Proceedings of the Xle colloque Elements Transposables, Montpellier, France.
Capy, P., C., Bazin, D. Higuet & Th. Langin, 1998 (Eds.). Dynamics and Evolution of Transposable Elements Springer, Landes Biosciences, Library of Congress, Austin, Texas.
Casa. A.M., C. Brouwer, A. Nagel, L. Wang, Q. Zhang, S. Kresovich & S.R. Wessler, 2000. The MITE family HeartBreaker (Hbr): Molecular markers in maize. Proc Natl Acad Sci USA 97(18): 10083–10089.
Chantret, N., A. Cenci, F. Sabot, O. Anderson & J. Dubcovsky, 2004. Sequencing of the Triticum monococcum hardness locus reveals good microcolinearity with rice. Mol Genet Genomics 271: 377–386.
Cheng, C., S. Tsuchimoto, H. Ohtsubo, E. Ohtsubo, 2000. Tnr8, a foldback transposable element from rice. Genes Genet Syst 75: 327–333.
Chopra, S., V. Brendel, J. Zhang, A.D. Axtell & Th. Peterson, 1999. Molecular characterization of a mutable pigmentation phenotype and isolation of the first active transposable element from Sorghum bicolor. Proc Natl Acad Sci USA 96(26): 15330-15335.
Dawson, A., E. Hartswood, T. Paterson & D.J. Finnegan, 1997. A LINE-like transposable element in Drosophila, the I factor, encodes a protein with properties similar to those of retroviral nucleocapsids. EMBO J 16(14): 4448–4455.
Deininger, P.L., H. Tiedge, J. Kim & J. Brosius, 1996. Evolution, expression, and possible function of a master gene for amplification of an interspersed repeated DNA family in rodents. Prog Nucleic Acid Res Mol Biol 52: 67–88.
Deragon, J.M., N. Gilbert, L. Rouquet, A. Lenoir, Ph. Arnaud & G. Picard, 1996. A transcriptional analysis of the Slsn (Brassica napus) family of SINE retroposons. Plant Mol Biol 32: 869–878.
Deragon, J.M., B.S. Landry, T. Pelissier, S. Tutois, S. Tourmente & G. Picard, 1994. An analysis of retroposition in plants based on a family of SINEs from Brassica napus. J Mol Evol 39: 378–386.
Devos, K.M., J.K.M. Brown & J.L. Bennetzen, 2002. Genome size reduction through illegitimate recombination counteracts genome expansion in Arabidopsis. Genome Res 12: 1075–1079.
Dong, F., J.T. Miller, S.A. Jackson, G.-L. Wang, P.C. Ronald & J. Jiang, 1998. Rice (Oryza sativd) centromeric regions consist of complex DNA. Proc Natl Acad Sci USA 95: 8135–8140.
Doolittle, W.F. & C. Sapienza, 1980. Selfish genes, the phenotype paradigm and genome evolution. Nature 284: 601–603.
Eickbush, T.H., 1999. Exon shuffling in retrospect. Science 283: 1465–1467.
Elrouby, N. & T.E. Bureau, 2001. A novel hybrid ORF formed by multiple cellular gene transductions by a plant LTR-retroelement. J Biol Chem 276(45): 41963–41968.
Fedoroff, N.V., 2000. Transposons and genome evolution in plants. Proc Natl Acad Sci USA 97(13): 7002–7007.
Fedoroff, N.V., S.R. Wessler & M. Shure, 1983. Isolation of the transposable maize controlling elements Ac and Ds. Cell 35(1): 235–242.
Feschotte, C., L. Swamy & S.R. Wessler, 2003. Genome-wide Analysis of mariner-like transposable elements in rice reveals complex relationships with Stowaway MITEs. Genetics 163: 747–758
Feschotte, C. & S.R., Wessler, 2001. Treasures in the attic: Rolling circle transposons discovered in eukaryotic genomes. Proc Natl Acad Sci USA 98(16): 8923–8924.
Flavell, A.J., 1999. Long terminal repeat retrotransposons jump between species. Proc Natl Acad Sci USA 96(22): 12211-12212.
Flavell, A.J., D.B. Smith & A. Kumar, 1992. Extreme heterogeneity of Copia-Ty family retrotransposons in plants. Mol Gen Genet 231(2): 233–242.
Flavell, R.B., J. Rimpau & D.B. Smith, 1977. Repeated sequence DNA relationship in four cereals genomes. Chromosoma 63: 205–222
Fukui, K.-N., G. Suzuki, E.S. Lagudah, S. Rahman, R. Appels, M. Yamamoto & Y. Mukai, 2001. Physical arrangement of retrotransposon-related repeats in centromeric regions of wheat. Plant Cell Physiol 42(2): 189–196.
Gabriel, A., M. Willems, E.H. Mules & J.D. Boeke, 1996. Replication infidelity during a single cycle of Ty retrotransposition. Proc. Natl. Acad. Sci. USA 93: 7767–71
M.J. Giroux, M. Clancy, J. Baier, L. Ingham, D. McCarty & L.C. Hannah, 1994. De novo synthesis of an intron by the maize transposable element Dissociation. Proc. Natl. Acad. Sci. USA 91: 12150–12154.
Goodwin, T.J.D. & R.T.M. Poulter, 2001. The DIRS 1 group of retrotransposons. Mol Biol Evol 18(11): 2067–2082.
Harberd, N.P., R.B. Flavell & R.D. Thompson, 1987. Identification of a transposon-like insertion in a Glu-1 allele of wheat. Mol Gen Genet 209: 326–332.
Henikoff, S. & M.A. Matzke, 1997. Exploring and explaining epigenetic effects. Trends In Genetics 13(8): 293–295.
Hickey, D.A., 1982. Selfish DNA: A sexually-transmitted nuclear parasite. Genetics 101: 519–531.
Hirochika, H., 1995. Activation of plant retrotransposons by stress. In: Modification of Gene Expression and Non-Mendelian Inheritance. NIAR, Japan, pp. 15–21.
Hu, J., V.S. Reddy & S.R. Wessler, 2000. The rice R gene family: Two distinct subfamilies containing several miniature inverted-repeat transposable elements. Plant Mol Biol 42: 667–678.
Hull, R., 1999. Classification of reverse transcribing elements: A discussion document. Arch Virol 144(1): 209–214.
Iwamoto, M. & K. Higo, 2003. Tourist C transposable elements are closely associated with genes expressed in flowers in rice (Oryza sativa). Mol Genet Genomics 268: 771–778.
Jääskeläinen, M.J., A.H. Mykkanen, T. Arna, C.M. Vicient, A. Suoniemi, R. Kalendar, H. Savilahti & A.H. Schulman, 1999. Retrotransposon BARE-1: Expression of encoded proteins and formation of virus-like particles in barley cells. Plant J 20(4): 413–422.
Jiang, N., Z., Bao, S., Temnykh, Z., Cheng, J., Jiang, R.A., Wing, S.R. McCouch & S.R., Wessler, 2002. Dasheng: A recently amplified nonautonomous LTR element that is a major component of pericentromeric regions in rice. Genetics 161(3): 1293–1305.
Jiang, N., Z. Bao, X. Zhang, H. Hirochika, S.R. Eddy, S.R. McCouch & S.R. Wessler, 2003. An active DNA transposon family in rice. Nature 421: 163–167.
Jiang, N., K. Jordan & S.R. Wessler, 2002. Dasheng and RIRE2. A nonautonomous long terminal repeat element and its putative autonomous partner in the rice genome. Plant Physiol 130: 1697–1705.
Jiang, N. & S.R. Wessler, 2001. Insertion preference of maize and rice miniature inverted repeat transposable elements as revealed by the analysis of nested element. Plant Cell 13: 2553–2564.
Jordan, I.K., L.V. Matyunina & J.F. McDonald, 1999. Evidence for the recent horizontal transfer of long terminal repeat retrotransposon. Proc Natl Acad Sci USA 96(22): 12621–12625.
Jurka, J., 1997. Sequence patterns indicate an enzymatic involvement in integration of mammalian retroposons. Proc Natl Acad Sci USA 94: 1872–1877.
Jurka, J., 1998. Repeats in genomic DNA: Mining and meaning. Curr Opin Struct Biol 8: 333–337.
Kalendar, R., J. Tanskanen, S. Immonen, E. Nevo & A.H. Schulman, 2000. Genome evolution of wild barley (Hordeum spontaneum) by BARE-1 retrotransposon dynamics in response to sharp microclimatic divergence. Proc Natl Acad Sci USA 97(12): 6603–6607.
Kalendar, R., C.M. Vicient, O. Peleg, Anamthawat-K. Jonsson, A. Bolshoy & A.H. Schulman, 2004. LArge Retrotransposon Derivatives: Abundant, conserved but nonautonomous retroelements of Barley and related genomes. Genetics 166: 1437–1450.
Kapitonov, V.V. & J. Jurka, 2001. Rolling-circle transposons in eukaryotes. Proc Natl Acad Sci USA 98(15): 8714–8719.
Kashkush, K., M. Feldman & A.A. Levy, 2002. Gene loss, silencing and activation in a newly synthesized wheat allotetraploid. Genetics 160: 1651–1659.
Kashkush, K., M. Feldman & A.A. Levy, 2003. Transcriptional activation of retrotransposons alters the expression of adjacent genes in wheat. Nat Genet 33: 102–106.
Kempken, F. & F. Windhofer, 2001. The hAT family: A versatile transposon group. Chromosoma 110: 1–9.
Kidwell, M.G. & D. Lisch, 1997. Transposable elements as sources of variation in animals and plants. Proc Natl Acad Sci USA 94: 7704–7711.
Kikuchi, K., K. Terauchi, M. Wada & H.-Y. Hirano, 2003. The plant MITE mPING is mobilized in anther culture. Nature 421: 167–170.
Kishii, M., K. Nagaki & H. Tsujimoto, 2001. A tandem repetitive sequence located in the centromeric region of common wheat (Triticum aestivum) chromosomes. Chromosome Res 9: 417–428.
Kloeckener-Gruissem, B. & M. Freeling, 1995. Transposon-induced promoter scrambling: A mechanism for the evolution of new alleles. Proc Natl Acad Sci USA 92: 1836–1840.
Kolosha, V.O. & S.L. Martin, 1997. In vitro properties of the first ORF protein from mouse LINE-1 support its role in ribonucleoprotein particle formation during retrotransposition. Proc Natl Acad Sci USA 94: 10155–10160.
Kumar, A. & J.L. Bennetzen, 1999. Plant retrotransposons. Annu Rev Genet 33: 479–532.
Kumar, A. & J.L. Bennetzen, 2000. Retrotransposons: Central players in the structure, evolution and function of plant genomes. Trends Plant Sci 5(12): 509–510.
Kumar, A. & H. Hirochika, 2001. Applications of retrotransposons as genetics tools in plants biology. Trends Plant Sci 6(3): 127-134.
Kumekawa, N., E. Ohtsubo & H. Ohtsubo, 1999. Identification and phylogenetic analysis of Gypsy-type retrotransposons in the plant kingdom. Genes Genet Syst 74: 299–307.
Lal, S.K., M.J. Giroux, V. Brendel, C.E. Vallejos & L.C. Hannah, 2003. The maize genome contains a Helitron insertion. Plant Cell 15: 381–391.
Langdon, T., G. Jenkins, R. Hasterok & I.P. King, 2003. A high-copy-number CACTA family transposon in temperate grasses and cereals. Genetics 163: 1097–1108.
Lawson, E.J.R., S.R. Scofield, C. Sjodin, J.D.G. Jones & C. Dean, 1994. Modification of the 5′ untranslated leader region of the maize Activator element leads to increased activity in Arabidopsis. Mol Gen Genet 245: 608–615.
Lenoir, A., L. Lavie, J.-L. Prieto, C. Goubely, J.-C. Cote, T. Pelissier & J.M. Deragon, 2001. The evolutionary origin and genomic organization of SINEs in Arabidopsis thaliana. Mol Biol Evol 18(12): 2315–2322.
Mahillon, J. & M. Chandler, 1998. Insertion sequences. Microbiol Mol Biol Rev 62(3): 725–774.
Malik, H.S. & T.H. Eickbush, 2001. Phylogenetic analysis of ribonuclease H domains suggests a late, chimeric origin of LTR retrotransposable element and retroviruses. Genome Res 11: 1187–1197.
Matsukoa, Y. & K. Tsunewaki, 1996. Wheat retrotransposon families identified by reverse transcriptase domain analysis. Mol Biol Evol 13(10): 1384–1392.
Matyunina, L.V., I.K. Jordan & J.F. McDonald, 1996. Naturally occuring variation in Copia expression is due to both element (cis) and host (trans) regulatory variation. Proc Natl Acad Sci USA 93: 7097–7102.
McClintock, B., 1950. Mutable loci in maize. In: Carnegie Institute of Washington Year Book, pp. 174–181, Washington.
McClintock, B., 1984. Significance of responses of the genome to challenge. Science 226: 792–801.
Miller, J.T., F. Dong, S.A. Jackson, J. Song & J. Jiang, 1998. Retrotransposon-related DNA sequences in the centromeres of grass chromosomes. Genetics 150: 1615–1623.
Mizuuchi, K., 1992. Transpositional recombination: Mechanistic insights from studies of Mu and other elements. Ann Rev Biochem 61: 1011–1051.
Modolell, J., W. Bender & M. Meleson, 1983. Drosophila melanogaster mutations suppressible by the suppressor of Hairy-wing are insertions of a 7.3-kilobases mobile element. Proc Natl Acad Sci USA 80(6): 1678–1682.
Moore, J.K. & J.E. Haber, 1996. Capture of retrotransposon DNA at the sites of chromosomal double-strand breaks. Nature 383: 644–646.
Moran, J.V., R.J. DeBernardinis & H.H. Kazazian, Jr., 1999. Exon shuffling by LI retrotransposition. Science 283: 1530–1534.
Mount, S.M. & G.M. Rubin, 1985. Complete nucleotide sequence of the Drosophila transposable element Copia: Homology between Copia and retroviral proteins. Mol Cell Biol 5(7): 1630–1638.
Nakayashiki, H., K. Ikeda, Y. Hashimoto, Y. Tosa & S. Mayama, 2001. Methylation is not the main force repressing the retrotransposon MAGGY in Magnaporthe grisea. Nucleic Acids Res 29(6): 1278–1284.
Nevo, E., 2001. Evolution of genome-phenome diversity under environmental stress. Proc Natl Acad Sci USA 98(11): 6233–6240.
Okamoto, H. & H. Hirochika, 2001. Silencing of transposable element in plants. Trends Plant Sci 6(11): 527–534.
Orgel, L.E. & H.C. Crick, 1980. Selfish DNA: The ultimate parasite. Nature 284: 604–607.
Pardue, M.L., 2000. Transposable elements: Friends, foes, or merely fellow travelers? Trends Genet 16(4): 155–156.
Pastink, A., J.C.J. Eeken & P.H.M. Lohman, 2001. Genomic integrity and the repair of double-strand DNA breaks. Mut Res 480–481: 37–50.
Pelissier, T., S. Tutois, J.M. Deragon, S. Tourmente, S. Genestier & G. Picard, 1995. Athila, a new retroelement from Arabidopsis thaliana. Plant Mol Biol 29(3): 441–452.
Petrov, D.A., 2001. Evolution of genome size: New approaches to an old problem. Trends Genet 17(1): 23–28.
Potter, S.S., 1982. DNA sequence analysis of a Drosophila foldback transposable element rearrangement. Mol Gen Genet 188(1): 107–110.
Presting, G.G., L. Malysheva, J. Fuchs & I. Schubert, 1998. A Ty3/Gypsy retrotransposon-like sequence localizes to the centromeric regions of cereal chromosomes. Plant J 16(6): 721–728.
Preston, B.D., 1996. Error-prone retrotransposition: Rime of the ancient mutators. Proc Natl Acad Sci USA 93: 7427–7431.
Purugganan, M.D. & S.R. Wessler, 1994. Molecular evolution of Magellan, a maize Ty3/Gypsy-like retrotransposon. Proc Natl Acad Sci USA 91: 11674–11678.
Rabinowicz, P.D., 2000. Are obese plant genome on a diet? Genome Res 10: 893–894.
Raina, R., D. Cook & N.V. Fedoroff, 1993. Maize Spm transposable element has an enhancer-insensitive promoter. Proc Natl Acad Sci USA 90: 6355–6359.
Rebatchouk, D. & J.O. Narita, 1997. Foldback transposable elements in plant. Plant Mol Biol 34(5): 831–835.
Rinehart, T.A., C. Dean & C.F., Weil, 1997. Comparative analysis of non-random DNA repair following Ac transposon excision in maize and Arabidopsis. Plant J 12(6): 1419–1427.
Rudenko, G.N., A. Ono & V. Walbot, 2003. Initiation of silencing of maize MuDR/Mu transposable elements. Plant J 33: 1013–1025.
Rudenko, G.N. & V. Walbot, 2001. Expression and post-transcriptional regulation of maize transposable element MuDR and its derivatives. Plant Cell 13: 553–570.
SanMiguel, Ph., A. Tikhonov, Y.-K. Jin, Motchoulskaia, N., D. Zakharov, A. Melake-Berhan, P.S. Springer, K.J. Edwards, M. Lee, Z. Avramova & J.L. Bennetzen, 1996. Nested retrotransposons in the intergenic regions of the maize genome. Science 274: 765–768.
Schmidt, T., 1999. LINEs, SINEs and repetitive DNA: Non-LTR retrotransposons in plant genomes. Plant Mol Biol 40: 903-910.
Shirasu, K., A.H. Schulman, T. Lahaye & P. Schulze-Lefert, 2000. A contiguous 66-kb barley DNA sequence provides evidence for reversible genome expansion. Genome Res 10(7): 908–915.
Suoniemi, A., K. Anamthawat-Jonsson, T. Arna & A.H. Schulman, 1996. Retrotransposon BARE-1 is a major, dispersed component of the barley (Hordeum vulgare L.) genome. Plant Mol Biol 30: 1321–1329.
Takasaki, N., S. Murata, M. Saitoh, T. Kobayashi, L. Park & N. Okada, 1994. Species-specific amplification of tRNA-derived short interspersed repetitive elements (SINEs) by retroposition: A process of parazitation of entire genome during the evolution of salmonides. Proc Natl Acad Sci USA 91: 10153–10157.
Temin, H.M., 1980. Origin of retro viruses from cellular moveable genetic elements. Cell 21: 599–600.
Thompson-Stewart, D., G.H. Karpen & A.C. Spradling, 1994. A transposable element can drive the concerted evolution of tandemly repetitious DNA. Proc Natl Acad Sci USA 91: 9042–9046.
Tikhonov, A., L. Lavie, Tatout, Ch., J.L. Bennetzen, Z. Avramova & J.M. Deragon, 2001. Target sites for SINE integration in Brassica genomes display nuclear matrix binding activity. Chromosome Res 9: 325–337.
Turcotte, K., S. Srinivasan & T.E. Bureau, 2001. Survey of transposable element from rice genomic sequences. Plant J 25(2): 169–179.
Vitte, C. & O. Panaud, 2003. Formation of Solo-LTRs through unequal homologous recombination counterbalances amplifications of LTR retrotransposons. Mol Biol Evol 20(4): 528–540.
Volff, J.-N., U. Hornung & M. Schartl, (2001a). Fish retroposons related to the Penelope element of Drosophila virilis define a new group of retrotransposable elements. Mol Genet Genomics 265: 711–720.
Volff, J.-N., C. Korting, A. Froschauer, K. Sweeney & M. Scharl, (2001b). Non-LTR retrotransposons encoding a restriction enzyme-like endonuclease in vertebrates. J Mol Evol 52: 351–360.
Walbot, V. & D.A. Petrov, 2001. Gene galaxies in the maize genome. Proc Natl Acad Sci USA 98(15): 8163–8164.
Waldrop, M., 1989. Did life really start out in an RNA world? Science 246(4935): 1248–1249.
Wendel, J.F. & S.R. Wessler, 2000. Retrotransposon-mediated genome evolution on a local ecological scale. Proc Natl Acad Sci USA 97(12): 6250–6252.
Wessler, S.R., 2001. Plant transposable element. A hard act to follow. Plant Physiol 125: 149–151.
Wessler, S.R., T.E. Bureau & S.E. White, 1995. LTR-retrotransposons and MITEs: Important players in the evolution of plant genomes. Curr Opin Genet Dev 5: 814–821.
White, S.E., L.F. Habera & S.R. Wessler, 1994. Retrotransposons in the flanking regions of normal plant genes: A role for Copza-like elements in the evolution of gene structure and expression. Proc Natl Acad Sci USA 91: 11792–11796.
Wicker, Th., R. Guyot, N. Yahiaoui &, B. Keller, 2003. CACTA transposon in Triticeae. A diverse family of high-copy repetitive elements. Plant Physiol 132: 52–63.
Witte, C.-P., Q.H. Le, T.E. Bureau & A. Kumar, 2001. Terminal-repeat retrotransposons in miniature (TRIM) are involved in restructuring plant genomes. Proc Natl Acad Sci USA 98(24): 13778–13783.
Xiong, Y. & T.H., Eickbush, 1990. Origin and evolution of retroelements based upon their reverse transcriptase sequences. EMBO J 9(10): 3353–3362.
Yamanouchi, K., 2000. Potential risk of genotransplant-associated infections. Transplant Proc 32: 1155–1156.
Yang, G. & T. Hall, 2003. MDM-1 and MDM-2: Two Mwtator-derived MITE families in rice. J Mol Evol 56: 255–264.
Yasui, Y., S. Nasuda, Y. Matsukoa & T. Kawahara, 2001. The Au family, a novel short interspersed element (SINE) from Aegilops umbellulata. Theor Appl Genet 102: 463–470.
Yoder, J.A., C.P. Walsh & T.H. Bestor, 1997. Cytosine methylation and the ecology of intragenomic parasites. Trends Genet 13(8): 335–340.
Zhang, X., C. Feschotte, Q. Zhang, N. Jiang, W.B. Eggleston & S.R. Wessler, 2001. P instability factor: An active maize transposon system associated with the amplification of Tourist-like MITEs and a new superfamily of transposases. Proc Natl Acad Sci USA 98(22): 12572–12577.
Zhang, X., N. Jiang, C. Feschotte & S.R. Wessler, 2004. PIF- and Pong-like transposable elements: Distribution, evolution and relationship with Tourist-like miniature inverted repeat tranposable elements. Genetics 166: 971–986.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sabot, F., Simon, D. & Bernard, M. Plant transposable elements, with an emphasis on grass species. Euphytica 139, 227–247 (2004). https://doi.org/10.1007/s10681-004-3179-y
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10681-004-3179-y