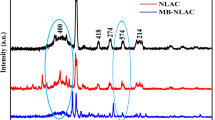
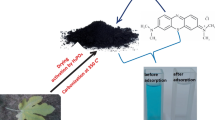
Abstract
Water bodies with the dye methylene blue pose serious environmental and health risks to humans. Therefore, the creation and investigation of affordable, potential adsorbents to remove methylene blue dye from water resources as a long-term fix is one focus of the scientific community. Food plants and other carbon-source serve as a hotspot for a wider range of application on different pollutants that impact the environment and living organisms. Here, we reviewed the use of treated and untreated biosorbents made from plant waste leaves for removing the dye methylene blue from aqueous media. After being modified, activated carbon made from various plant leaves improves adsorption performance. The range of activating chemicals, activation methods, and bio-sorbent material characterisation using FTIR analysis, Barunauer-Emmett-Teller (BET) surface area, scanning electron microscope (SEM-EDX), and SEM-EDX have all been covered in this review. It has been thoroughly described how the pH solution of the methylene blue dye compares to the pHPZC of the adsorbent surface. The presentation also includes a thorough analysis of the application of the isotherm model, kinetic model, and thermodynamic parameters. The selectivity of the adsorbent is the main focus of the adsorption kinetics and isotherm models. It has been studied how adsorption occurs, how surface area and pH affect it, and how biomass waste compares to other adsorbents. The use of biomass waste as adsorbents is both environmentally and economically advantageous, and it has been discovered to have exceptional color removal capabilities.











Similar content being viewed by others
Data availability
The dataset utilized/analyzed during the current study will be available from the corresponding author upon request.
References
Abdulraheem, G., Bala, S., Muhammad, S., & Abdullahi, M. (2015). Kinetics, equilibrium and thermodynamics studies of CI Reactive Blue 19 dye adsorption on coconut shell based activated carbon. International Biodeterioration & Biodegradation, 102, 265–273.
Adegoke, K. A., & Bello, O. S. (2015). Dye sequestration using agricultural wastes as adsorbents. Water Resources and Industry, 12, 8–24.
Affam, A. C. (2020). Conventional steam activation for conversion of oil palm kernel shell biomass into activated carbon via biochar product. Global Journal of Environmental Science and Management, 6(1), 15–30.
Aguayo-Villarreal, I. A., Bonilla-Petriciolet, A., & Muñiz-Valencia, R. (2017). Preparation of activated carbons from pecan nutshell and their application in the antagonistic adsorption of heavy metal ions. Journal of Molecular Liquids, 230, 686–695.
Ahmad Khan, F., & Farooqui, M. (2022). Removal of methylene blue dye from aqueous solutions onto Morus nigra L.(mulberry tree) leaves powder and its biochar—equilibrium, kinetic and thermodynamic study. International Journal of Environmental Analytical Chemistry, 1–20.
Ahmad, M. A., & Alrozi, R. (2011). Removal of malachite green dye from aqueous solution using rambutan peel-based activated carbon: Equilibrium, kinetic and thermodynamic studies. Chemical Engineering Journal, 171(2), 510–516.
Ahmed, M. J. (2016). Preparation of activated carbons from date (Phoenix dactylifera L.) palm stones and application for wastewater treatments. Process Safety and Environmental Protection, 102, 168–182.
Ahsan, M. A., Katla, S. K., Islam, M. T., Hernandez-Viezcas, J. A., Martinez, L. M., Díaz-Moreno, C. A., et al. (2018). Adsorptive removal of methylene blue, tetracycline and Cr (VI) from water using sulfonated tea waste. Environmental Technology & Innovation, 11, 23–40.
Ai, L., Zhang, C., Liao, F., Wang, Y., Li, M., Meng, L., & Jiang, J. (2011). Removal of methylene blue from aqueous solution with magnetite loaded multi-wall carbon nanotube: Kinetic, isotherm and mechanism analysis. Journal of Hazardous Materials, 198, 282–290.
Akar, E., Altinişik, A., & Seki, Y. (2013). Using of activated carbon produced from spent tea leaves for the removal of malachite green from aqueous solution. Ecological Engineering, 52, 19–27.
Al-Mokhalelati, K., Al-Bakri, I., & Al Shibeh Al Wattar, N. (2021). Adsorption of methylene blue onto sugarcane bagasse-based adsorbent materials. Journal of Physical Organic Chemistry, 34(7), e4193.
Alam, M. Z., & Faisal Anwar, A. H. M. (2020). Nutrients adsorption onto biochar and alum sludge for treating stormwater. Journal of Water and Environment Technology, 18(2), 132–146.
Alam, M. Z., Bari, M. N., & Kawsari, S. (2022). Statistical optimization of Methylene Blue dye removal from a synthetic textile wastewater using indigenous adsorbents. Environmental and Sustainability Indicators, 14, 100176.
Almasi, A., Navazeshkhaa, F., & Mousavi, S. A. (2017a). Biosorption of lead from aqueous solution onto Nasturtium officinale: Performance and modeling. Desalination and Water Treatment, 65, 443–450.
Almasi, A., Rostamkhani, Z., & Mousavi, S. A. (2017b). Adsorption of Reactive Red 2 using activated carbon prepared from walnut shell: Batch and fixed bed studies. Desalination and Water Treatment, 79, 356–367.
Arun, J., Gopinath, K. P., SundarRajan, P., Malolan, R., & AjaySrinivaasan, P. (2020). Hydrothermal liquefaction and pyrolysis of Amphiroa fragilissima biomass: Comparative study on oxygen content and storage stability parameters of bio-oil. Bioresource Technology Reports, 11, 100465.
Aslam, M. S., Ahmad, M. S., & Mamat, A. S. (2015). A phytochemical, ethnomedicinal and pharmacological review of genus Dipterocarpus. International Journal of Pharmacy and Pharmaceutical Sciences, 7(4), 27–38.
Auta, M., & Hameed, B. H. (2011a). Preparation of waste tea activated carbon using potassium acetate as an activating agent for adsorption of Acid Blue 25 dye. Chemical Engineering Journal, 171(2), 502–509.
Auta, M., & Hameed, B. H. (2011b). Optimized waste tea activated carbon for adsorption of Methylene Blue and Acid Blue 29 dyes using response surface methodology. Chemical Engineering Journal, 175, 233–243.
Azoulay, K., Bencheikh, I., Moufti, A., Dahchour, A., Mabrouki, J., & El Hajjaji, S. (2020). Comparative study between static and dynamic adsorption efficiency of dyes by the mixture of palm waste using the central composite design. Chemical Data Collections, 27, 100385.
Azzaz, A. A., Jellali, S., Assadi, A. A., & Bousselmi, L. (2016). Chemical treatment of orange tree sawdust for a cationic dye enhancement removal from aqueous solutions: Kinetic, equilibrium and thermodynamic studies. Desalination and Water Treatment, 57(46), 22107–22119.
Barpanda, P., Fanchini, G., & Amatucci, G. G. (2011). Structure, surface morphology and electrochemical properties of brominated activated carbons. Carbon, 49(7), 2538–2548.
Bayomie, O. S., Kandeel, H., Shoeib, T., Yang, H., Youssef, N., & El-Sayed, M. M. H. (2020). Novel approach for effective removal of methylene blue dye from water using fava bean peel waste. Scientific Reports, 10(1), 1–10.
Bello, O. S., Owojuyigbe, E. S., Babatunde, M. A., & Folaranmi, F. E. (2017). Sustainable conversion of agro-wastes into useful adsorbents. Applied Water Science, 7(7), 3561–3571.
Bencheikh, I., Azoulay, K., Mabrouki, J., El Hajjaji, S., Dahchour, A., Moufti, A., & Dhiba, D. (2020). The adsorptive removal of MB using chemically treated artichoke leaves: Parametric, kinetic, isotherm and thermodynamic study. Scientific African, 9, e00509.
Bharathi, K. S., & Ramesh, S. T. (2013). Removal of dyes using agricultural waste as low-cost adsorbents: A review. Applied Water Science, 3(4), 773–790.
Borhan, A., Abdullah, N. A., Rashidi, N. A., & Taha, M. F. (2016). Removal of Cu2+ and Zn2+ from single metal aqueous solution using rubber-seed shell based activated carbon. Procedia Engineering, 148, 694–701.
Borrell, J. S., Biswas, M. K., Goodwin, M., Blomme, G., Schwarzacher, T., Heslop-Harrison, J. S., et al. (2019). Enset in Ethiopia: A poorly characterized but resilient starch staple. Annals of Botany, 123(5), 747–766.
Borrell, J. S., Goodwin, M., Blomme, G., Jacobsen, K., Wendawek, A. M., Gashu, D., et al. (2020). Enset-based agricultural systems in Ethiopia: A systematic review of production trends, agronomy, processing and the wider food security applications of a neglected banana relative. Plants, People, Planet, 2(3), 212–228.
Bounaas, M., Bouguettoucha, A., Chebli, D., Gatica, J. M., & Vidal, H. (2021). Role of the wild carob as biosorbent and as precursor of a new high-surface-area activated carbon for the adsorption of methylene blue. Arabian Journal for Science and Engineering, 46, 325–341.
Canales-Flores, R. A., & Prieto-García, F. (2020). Taguchi optimization for production of activated carbon from phosphoric acid impregnated agricultural waste by microwave heating for the removal of methylene blue. Diamond and Related Materials, 109, 108027.
Chen, C. J., Jiang, R., Wang, G., Jiao, R. H., Tancharoen, C., Sudto, K., et al. (2014). Oligostilbenoids with acetylcholinesterase inhibitory activity from Dipterocarpus alatus. Planta Medica, 80(17), 1641–1646.
Chen, J., Wang, X., Huang, Y., Lv, S., Cao, X., Yun, J., & Cao, D. (2018). Adsorption removal of pollutant dyes in wastewater by nitrogen-doped porous carbons derived from natural leaves. Engineered Science, 5(11), 30–38.
Chen, K., & Lu, Z. (2018). Immune responses to bacterial and fungal infections in the silkworm, Bombyx mori. Developmental & Comparative Immunology, 83, 3–11.
Cheng, Z., Zhang, L., Guo, X., Jiang, X., & Liu, R. (2015). Removal of Lissamine rhodamine B and acid orange 10 from aqueous solution using activated carbon/surfactant: Process optimization, kinetics and equilibrium. Journal of the Taiwan Institute of Chemical Engineers, 47, 149–159.
Chowdhury, S., Chakraborty, S., & Saha, P. (2011). Biosorption of Basic Green 4 from aqueous solution by Ananas comosus (pineapple) leaf powder. Colloids and Surfaces b: Biointerfaces, 84(2), 520–527.
Dada, A. O., Olalekan, A. P., Olatunya, A. M., & Dada, O. (2012). Langmuir, Freundlich, Temkin and Dubinin-Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR Journal of Applied Chemistry, 3(1), 38–45.
Dardouri, S., & Sghaier, J. (2017). Adsorptive removal of methylene blue from aqueous solution using different agricultural wastes as adsorbents. Korean Journal of Chemical Engineering, 34(4), 1037–1043.
Dbik, A., El Messaoudi, N., Bentahar, S., El Khomri, M., Lacherai, A., & Faska, N. (2022). Optimization of methylene blue adsorption on agricultural solid waste using box–behnken design (BBD) combined with response surface methodology (RSM) modeling. Biointerface Research in Applied Chemistry, 12(4), 4567–4583.
de Carvalho, H. P., Huang, J., Zhao, M., Liu, G., Dong, L., & Liu, X. (2015). Improvement of Methylene Blue removal by electrocoagulation/banana peel adsorption coupling in a batch system. Alexandria Engineering Journal, 54(3), 777–786.
De Marco, C., Mauler, R. S., Daitx, T. S., Krindges, I., Cemin, A., Bonetto, L. R., et al. (2020). Removal of malachite green dye from aqueous solutions by a magnetic adsorbent. Separation Science and Technology, 55(6), 1089–1101.
Dehghani, M. H., Karri, R. R., Yeganeh, Z. T., Mahvi, A. H., Nourmoradi, H., Salari, M., et al. (2020). Statistical modelling of endocrine disrupting compounds adsorption onto activated carbon prepared from wood using CCD-RSM and DE hybrid evolutionary optimization framework: Comparison of linear vs non-linear isotherm and kinetic parameters. Journal of Molecular Liquids, 302, 112526.
Demeke, T. (1986). Is Ethiopia’s Ensete ventricosum crop her greatest potential food. Agriculture International, 38, 362–365.
Devi, R., Singh, V., & Kumar, A. (2008). COD and BOD reduction from coffee processing wastewater using Avacado peel carbon. Bioresource Technology, 99(6), 1853–1860.
Dinh, V.-P., Le, H. M., Nguyen, V.-D., Dao, V.-A., Hung, N. Q., Tuyen, L. A., et al. (2019). Insight into the adsorption mechanisms of methylene blue and chromium (III) from aqueous solution onto pomelo fruit peel. RSC Advances, 9(44), 25847–25860.
Dos Santos, K. J. L., Dos Santos, G. E. de S., de Sá, Í. M. G. L., Ide, A. H., da Silva Duarte, J. L., de Carvalho, S. H. V., et al. (2019). Wodyetia bifurcata biochar for methylene blue removal from aqueous matrix. Bioresource Technology, 293, 122093.
Dotto, Guilherme L, Sharma, S. K., & Pinto, L. A. A. (2015). Biosorption of organic dyes: research opportunities and challenges. Green chemistry for dyes removal from wastewater, 295–329.
Dotto, Guilherme Luiz, Salau, N. P. G., Piccin, J. S., Cadaval, T. R. S., & Pinto, L. A. A. de. (2017). Adsorption kinetics in liquid phase: Modeling for discontinuous and continuous systems. In Adsorption processes for water treatment and purification, 53–76.
Duan, X., Srinivasakannan, C., Wang, X., Wang, F., & Liu, X. (2017). Synthesis of activated carbon fibers from cotton by microwave induced H3PO4 activation. Journal of the Taiwan Institute of Chemical Engineers, 70, 374–381.
Dural, M. U., Cavas, L., Papageorgiou, S. K., & Katsaros, F. K. (2011). Methylene blue adsorption on activated carbon prepared from Posidonia oceanica (L.) dead leaves: Kinetics and equilibrium studies. Chemical Engineering Journal, 168(1), 77–85.
El Messaoudi, N., El Khomri, M., Goodarzvand Chegini, Z., Chlif, N., Dbik, A., Bentahar, S., et al. (2021). Desorption study and reusability of raw and H2SO4 modified jujube shells (Zizyphus lotus) for the methylene blue adsorption. International Journal of Environmental Analytical Chemistry, 1–17.
El Messaoudi, N., El Mouden, A., El Khomri, M., Bouich, A., Fernine, Y., Ciğeroğlu, Z., et al. (2022). Experimental study and theoretical statistical modeling of acid blue 25 remediation using activated carbon from Citrus sinensis leaf. Fluid Phase Equilibria, 563, 113585.
Elessawy, N. A., El-Sayed, E. M., Ali, S., Elkady, M. F., Elnouby, M., & Hamad, H. A. (2020). One-pot green synthesis of magnetic fullerene nanocomposite for adsorption characteristics. Journal of Water Process Engineering, 34, 101047.
Elhussien, M. H., Hussein, R. M., Nimir, S. A., & Elsaim, M. H. (2017). Preparation and characterization of activated carbon from palm tree leaves impregnated with zinc chloride for the removal of lead (II) from aqueous solutions. American Journal of Physical Chemistry, 6(4), 59–69.
Escudero, L. B., Quintas, P. Y., Wuilloud, R. G., & Dotto, G. L. (2019). Recent advances on elemental biosorption. Environmental Chemistry Letters, 17(1), 409–427.
Farhadi, A., Ameri, A., & Tamjidi, S. (2021). Application of agricultural wastes as a low-cost adsorbent for removal of heavy metals and dyes from wastewater: A review study. Physical Chemistry Research, 9(2), 211–226.
Fathy, N. A., El-Shafey, O. I., & Khalil, L. B. (2013). Effectiveness of alkali-acid treatment in enhancement the adsorption capacity for rice straw: the removal of methylene blue dye. International Scholarly Research Notices, 2013, 1–15.
Feng, X., He, Y., Fang, J., Fang, Z., Jiang, B., Hulmel, M. B., et al. (2015). Comparison of the growth and biomass production of Miscanthus sinensis, Miscanthus floridulus and Saccharum arundinaceum. Spanish Journal of Agricultural Research, 13(3), e0703.
Ferruzzi, M. G. (2010). The influence of beverage composition on delivery of phenolic compounds from coffee and tea. Physiology & Behavior, 100(1), 33–41.
Foo, K. Y., & Hameed, B. H. (2012). Textural porosity, surface chemistry and adsorptive properties of durian shell derived activated carbon prepared by microwave assisted NaOH activation. Chemical Engineering Journal, 187, 53–62.
Freundlich, H. (1907). Ueber die adsorption in loesungen. Zeitschrift Für Physikalische Chemie, 57, 385–470.
Georgin, J., da Silva Marques, B., da Silveira Salla, J., Foletto, E. L., Allasia, D., & Dotto, G. L. (2018). Removal of Procion Red dye from colored effluents using H2SO4-/HNO3-treated avocado shells (Persea americana) as adsorbent. Environmental Science and Pollution Research, 25(7), 6429–6442.
Ghaedi, M., Taghavimoghadam, N., Naderi, S., Sahraei, R., & Daneshfar, A. (2013). Comparison of removal of bromothymol blue from aqueous solution by multiwalled carbon nanotube and Zn (OH) 2 nanoparticles loaded on activated carbon: A thermodynamic study. Journal of Industrial and Engineering Chemistry, 19(5), 1493–1500.
Ghosh, K., Bar, N., Biswas, A. B., & Das, S. K. (2021). Elimination of crystal violet from synthetic medium by adsorption using unmodified and acid-modified eucalyptus leaves with MPR and GA application. Sustainable Chemistry and Pharmacy, 19, 100370.
Gündüz, F., & Bayrak, B. (2017). Biosorption of malachite green from an aqueous solution using pomegranate peel: Equilibrium modelling, kinetic and thermodynamic studies. Journal of Molecular Liquids, 243, 790–798.
Guo, D., Li, Y., Cui, B., Hu, M., Luo, S., Ji, B., & Liu, Y. (2020). Natural adsorption of methylene blue by waste fallen leaves of Magnoliaceae and its repeated thermal regeneration for reuse. Journal of Cleaner Production, 267, 121903.
Guo, J.-Z., Li, B., Liu, L., & Lv, K. (2014). Removal of methylene blue from aqueous solutions by chemically modified bamboo. Chemosphere, 111, 225–231.
Gupta, N., Kushwaha, A. K., & Chattopadhyaya, M. C. (2016). Application of potato (Solanum tuberosum) plant wastes for the removal of methylene blue and malachite green dye from aqueous solution. Arabian Journal of Chemistry, 9, S707–S716.
Halysh, V., Sevastyanova, O., Pikus, S., Dobele, G., Pasalskiy, B., Gun’ko, V. M., & Kartel, M. (2020). Sugarcane bagasse and straw as low-cost lignocellulosic sorbents for the removal of dyes and metal ions from water. Cellulose, 27(14), 8181–8197.
Hamad, H. N., & Idrus, S. (2022). Recent developments in the application of bio-waste-derived adsorbents for the removal of methylene blue from wastewater: A review. Polymers, 14(4), 783.
Hameed, B. H. (2009). Spent tea leaves: A new non-conventional and low-cost adsorbent for removal of basic dye from aqueous solutions. Journal of Hazardous Materials, 161(2–3), 753–759.
Han, R., Zou, W., Yu, W., Cheng, S., Wang, Y., & Shi, J. (2007). Biosorption of methylene blue from aqueous solution by fallen phoenix tree’s leaves. Journal of Hazardous Materials, 141(1), 156–162.
Hesas, R. H., Daud, W. M. A. W., Sahu, J. N., & Arami-Niya, A. (2013). The effects of a microwave heating method on the production of activated carbon from agricultural waste: A review. Journal of Analytical and Applied Pyrolysis, 100, 1–11.
Immich, A. P. S., de Souza, A. A. U., & Ulson, S. M. A. G. (2009). Removal of Remazol Blue RR dye from aqueous solutions with Neem leaves and evaluation of their acute toxicity with Daphnia magna. Journal of Hazardous Materials, 164(2–3), 1580–1585.
Ioannidou, O., & Zabaniotou, A. (2007). Agricultural residues as precursors for activated carbon production—a review. Renewable and Sustainable Energy Reviews, 11(9), 1966–2005.
Jawad, A. H., Abdulhameed, A. S., Hanafiah, M., ALOthman, Z. A., Khan, M. R., & Surip, S. N. (2021). Numerical desirability function for adsorption of methylene blue dye by sulfonated pomegranate peel biochar: Modeling, kinetic, isotherm, thermodynamic, and mechanism study. Korean Journal of Chemical Engineering, 38(7), 1499–1509.
Jawad, A. H., Abdulhameed, A. S., Wilson, L. D., Syed-Hassan, S. S. A., ALOthman, Z. A., & Khan, M. R. (2021). High surface area and mesoporous activated carbon from KOH-activated dragon fruit peels for methylene blue dye adsorption: Optimization and mechanism study. Chinese Journal of Chemical Engineering, 32, 281–290.
Jawad, A. H., Mallah, S. H., & Mastuli, M. S. (2018a). Adsorption behavior of methylene blue on acid-treated rubber (Hevea brasiliensis) leaf. Desalination and Water Treatment, 124, 297–307.
Jawad, A. H., Ramlah, A. R., Khudzir, I., & Sabar, S. (2017). High surface area mesoporous activated carbon developed from coconut leaf by chemical activation with H3PO4 for adsorption of methylene blue. Desalination and Water Treatment, 74, 326–335.
Jawad, A. H., Rashid, R. A., Ishak, M. A. M., & Wilson, L. D. (2016a). Adsorption of methylene blue onto activated carbon developed from biomass waste by H2SO4 activation: Kinetic, equilibrium and thermodynamic studies. Desalination and Water Treatment, 57(52), 25194–25206.
Jawad, A. H., Rashid, R. A., Mahmuod, R. M. A., Ishak, M. A. M., Kasim, N. N., & Ismail, K. (2016b). Adsorption of methylene blue onto coconut (Cocos nucifera) leaf: Optimization, isotherm and kinetic studies. Desalination and Water Treatment, 57(19), 8839–8853.
Jawad, A. H., Sauodi, M. H., Mastuli, M. S., Aouda, M. A., & Radzun, K. A. (2018b). Pomegranate peels collected from fresh juice shop as a renewable precursor for high surface area activated carbon with potential application for methylene blue adsorption. Desalination and Water Treatment, 124, 287–296.
Kamari, A., Yusoff, S. N. M., Abdullah, F., & Putra, W. P. (2014). Biosorptive removal of Cu (II), Ni (II) and Pb (II) ions from aqueous solutions using coconut dregs residue: Adsorption and characterisation studies. Journal of Environmental Chemical Engineering, 2(4), 1912–1919.
Karagöz, S., Tay, T., Ucar, S., & Erdem, M. (2008). Activated carbons from waste biomass by sulfuric acid activation and their use on methylene blue adsorption. Bioresource Technology, 99(14), 6214–6222.
Karnick, C. R., & Hocking, G. M. (1975). Ethnobotanical records of drug plants described in valmiki Ramayana and their uses in the ayurvedic system of medicine. Quarterly Journal of Crude Drug Research, 13(3–4), 143–154.
Katheresan, V., Kansedo, J., & Lau, S. Y. (2018). Efficiency of various recent wastewater dye removal methods: A review. Journal of Environmental Chemical Engineering, 6(4), 4676–4697.
Kezerle, A., Velic, N., Hasenay, D., & Kovacevic, D. (2018). Lignocellulosic materials as dye adsorbents: Adsorption of methylene blue and Congo red on Brewers’ spent grain. Croatica Chemica Acta, 91(1), 53–65.
Khan, T. A., & Nazir, M. (2015). Enhanced adsorptive removal of a model acid dye bromothymol blue from aqueous solution using magnetic chitosan-bamboo sawdust composite: Batch and column studies. Environmental Progress & Sustainable Energy, 34(5), 1444–1454.
Khan, T., Binti Abd Manan, T. S., Isa, M. H., Ghanim, A. A. J., Beddu, S., Jusoh, H., et al. (2020). Modeling of Cu (II) adsorption from an aqueous solution using an Artificial Neural Network (ANN). Molecules, 25(14), 3263.
Khangwichian, W., Pattamasewe, S., Laungphairojana, A., Leesing, R., Hunt, A. J., & Ngernyen, Y. (2021). Preparation of activated carbons from hydrolyzed Dipterocarpus alatus leaves: Value added product from biodiesel production waste. Journal of the Japan Institute of Energy, 100(10), 219–224.
Khangwichian, W., Pattamasewe, S., Leesing, R., Knijnenburg, J. T. N., & Ngernyen, Y. (2022). Adsorption of cationic dye on activated carbon from hydrolyzed Dipterocarpus alatus leaves: Waste from biodiesel production. Engineering and Applied Science Research, 49(4), 531–544.
Khodaie, M., Ghasemi, N., Moradi, B., & Rahimi, M. (2013). Removal of methylene blue from wastewater by adsorption onto ZnCl2 activated corn husk carbon equilibrium studies. Journal of Chemistry, 2013, 1–6.
Khomri, M. E., Messaoudi, N. E., Dbik, A., Bentahar, S., Fernine, Y., Bouich, A., et al. (2022). Modification of low-cost adsorbent prepared from agricultural solid waste for the adsorption and desorption of cationic dye. Emergent Materials, 5(6), 1679–1688.
Khurshid, H., Mustafa, M. R. U., & Isa, M. H. (2022). Modified activated carbon synthesized from oil palm leaves waste as a novel green adsorbent for chemical oxygen demand in produced water. Sustainability, 14(4), 1986.
King, P., Rakesh, N., Beenalahari, S., Kumar, Y. P., & Prasad, V. (2007). Removal of lead from aqueous solution using Syzygium cumini L.: equilibrium and kinetic studies. Journal of Hazardous Materials, 142(1–2), 340–347.
King, P., Rakesh, N., Lahari, S. B., Kumar, Y. P., & Prasad, V. (2008). Biosorption of zinc onto Syzygium cumini L.: Equilibrium and kinetic studies. Chemical Engineering Journal, 144(2), 181–187.
Krawczyk, K., & Łochyńska, M. (2020). Identification and characterization of Pseudomonas syringae pv. mori affecting white mulberry (Morus alba) in Poland. European Journal of Plant Pathology, 158(1), 281–291.
Krishni, R. R., Foo, K. Y., & Hameed, B. H. (2014). Adsorptive removal of methylene blue using the natural adsorbent-banana leaves. Desalination and Water Treatment, 52(31–33), 6104–6112.
Kumar, J. A., Amarnath, D. J., Sathish, S., Jabasingh, S. A., Saravanan, A., Hemavathy, R. V., et al. (2019). Enhanced PAHs removal using pyrolysis-assisted potassium hydroxide induced palm shell activated carbon: Batch and column investigation. Journal of Molecular Liquids, 279, 77–87.
Kushwaha, A. K., Gupta, N., & Chattopadhyaya, M. C. (2014). Removal of cationic methylene blue and malachite green dyes from aqueous solution by waste materials of Daucus carota. Journal of Saudi Chemical Society, 18(3), 200–207.
Lakkaboyana, S. K., Soontarapa, K., Marella, R. K., & Kannan, K. (2021). Preparation of novel chitosan polymeric nanocomposite as an efficient material for the removal of Acid Blue 25 from aqueous environment. International Journal of Biological Macromolecules, 168, 760–768.
Langmuir, I. (1918). The adsorption of gases on plane surfaces of glass, mica and platinum. Journal of the American Chemical Society, 40(9), 1361–1403.
Lata, H., Garg, V. K., & Gupta, R. K. (2007). Removal of a basic dye from aqueous solution by adsorption using Parthenium hysterophorus: An agricultural waste. Dyes and Pigments, 74(3), 653–658.
Lattanzio, V., Kroon, P. A., Linsalata, V., & Cardinali, A. (2009). Globe artichoke: A functional food and source of nutraceutical ingredients. Journal of Functional Foods, 1(2), 131–144.
Li, H., Li, Y., & Nie, Y. (2019). Research status of occurrence and control of Fusarium wilt of banana. Journal of South China Agricultural University, 40(5), 128–136.
Lim, W. C., Srinivasakannan, C., & Al Shoaibi, A. (2015). Cleaner production of porous carbon from palm shells through recovery and reuse of phosphoric acid. Journal of Cleaner Production, 102, 501–511.
Liu, F., Teng, S., Song, R., & Wang, S. (2010a). Adsorption of methylene blue on anaerobic granular sludge: Effect of functional groups. Desalination, 263(1–3), 11–17.
Liu, H., Dong, Y., Wang, H., & Liu, Y. (2010b). Ammonium adsorption from aqueous solutions by strawberry leaf powder: Equilibrium, kinetics and effects of coexisting ions. Desalination, 263(1–3), 70–75.
Liu, L., Gao, Z. Y., Su, X. P., Chen, X., Jiang, L., & Yao, J. M. (2015). Adsorption removal of dyes from single and binary solutions using a cellulose-based bioadsorbent. ACS Sustainable Chemistry & Engineering, 3(3), 432–442.
Low, L. W., Teng, T. T., Ahmad, A., Morad, N., & Wong, Y. S. (2011). A novel pretreatment method of lignocellulosic material as adsorbent and kinetic study of dye waste adsorption. Water, Air, & Soil Pollution, 218(1), 293–306.
Maan, A. A., Nazir, A., Khan, M. K. I., Ahmad, T., Zia, R., Murid, M., & Abrar, M. (2018). The therapeutic properties and applications of Aloe vera: A review. Journal of Herbal Medicine, 12, 1–10.
Madhav, S., Ahamad, A., Singh, P., & Mishra, P. K. (2018). A review of textile industry: Wet processing, environmental impacts, and effluent treatment methods. Environmental Quality Management, 27(3), 31–41.
Mahamad, M. N., Zaini, M. A. A., & Zakaria, Z. A. (2015). Preparation and characterization of activated carbon from pineapple waste biomass for dye removal. International Biodeterioration & Biodegradation, 102, 274–280.
Malakootian, M., & Heidari, M. R. (2018). Reactive orange 16 dye adsorption from aqueous solutions by psyllium seed powder as a low-cost biosorbent: Kinetic and equilibrium studies. Applied Water Science, 8(7), 1–9.
Mangla, D., Sharma, A., & Ikram, S. (2022). Synthesis of ecological chitosan/PVP magnetic composite: Remediation of amoxicillin trihydrate from its aqueous solution, isotherm modelling, thermodynamic, and kinetic studies. Reactive and Functional Polymers, 175, 105261.
Manna, S., Roy, D., Saha, P., Gopakumar, D., & Thomas, S. (2017). Rapid methylene blue adsorption using modified lignocellulosic materials. Process Safety and Environmental Protection, 107, 346–356.
Martín-González, M. A., Susial, P., Pérez-Peña, J., & Doña-Rodríguez, J. M. (2013). Preparation of activated carbons from banana leaves by chemical activation with phosphoric acid. Adsorption of methylene blue. Revista Mexicana de Ingeniería Química, 12(3), 595–608.
Mekuria, D., Diro, A., Melak, F., & Asere, T. G. (2022). Adsorptive removal of methylene blue dye using biowaste materials: barley bran and enset midrib leaf. Journal of Chemistry, 2022, 1–13.
Mohan, D., Singh, K. P., & Singh, V. K. (2008). Wastewater treatment using low cost activated carbons derived from agricultural byproducts—a case study. Journal of Hazardous Materials, 152(3), 1045–1053.
Montañez-Soto, J., Venegas-González, J., Vivar-Vera, M., & Ramos-Ramírez, E. (2011). Extracción, caracterización y cuantificación de los fructanos contenidos en la cabeza y en las hojas del Agave tequilana Weber azul. Bioagro, 23(3), 199–206.
Mousavi, S. A., Mahmoudi, A., Amiri, S., Darvishi, P., & Noori, E. (2022). Methylene blue removal using grape leaves waste: Optimization and modeling. Applied Water Science, 12(5), 1–11.
Nasrullah, A., Bhat, A. H., Naeem, A., Isa, M. H., & Danish, M. (2018). High surface area mesoporous activated carbon-alginate beads for efficient removal of methylene blue. International Journal of Biological Macromolecules, 107, 1792–1799.
Nekouei, F., Nekouei, S., Tyagi, I., & Gupta, V. K. (2015). Kinetic, thermodynamic and isotherm studies for acid blue 129 removal from liquids using copper oxide nanoparticle-modified activated carbon as a novel adsorbent. Journal of Molecular Liquids, 201, 124–133.
Nie, Z.-L., Wen, J., Azuma, H., Qiu, Y.-L., Sun, H., Meng, Y., et al. (2008). Phylogenetic and biogeographic complexity of Magnoliaceae in the Northern Hemisphere inferred from three nuclear data sets. Molecular Phylogenetics and Evolution, 48(3), 1027–1040.
Nieto-Delgado, C., Terrones, M., & Rangel-Mendez, J. R. (2011). Development of highly microporous activated carbon from the alcoholic beverage industry organic by-products. Biomass and Bioenergy, 35(1), 103–112.
Nithyalakshmi, B., & Saraswathi, R. (2023). Removal of colorants from wastewater using biochar derived from leaf waste. Biomass Conversion and Biorefinery, 13, 311–1327.
Njoku, V. O., Islam, M. A., Asif, M., & Hameed, B. H. (2014). Preparation of mesoporous activated carbon from coconut frond for the adsorption of carbofuran insecticide. Journal of Analytical and Applied Pyrolysis, 110, 172–180.
Nzikou, J. M., Kimbonguila, A., Matos, L., Loumouamou, B., Pambou-Tobi, N. P. G., Ndangui, C. B., et al. (2010). Extraction and characteristics of seed kernel oil from mango (Mangifera indica). Research Journal of Environmental and Earth Sciences, 2(1), 31–35.
Omo-Okoro, P. N., Daso, A. P., & Okonkwo, J. O. (2018). A review of the application of agricultural wastes as precursor materials for the adsorption of per-and polyfluoroalkyl substances: A focus on current approaches and methodologies. Environmental Technology & Innovation, 9, 100–114.
Ozdemir, I., Şahin, M., Orhan, R., & Erdem, M. (2014). Preparation and characterization of activated carbon from grape stalk by zinc chloride activation. Fuel Processing Technology, 125, 200–206.
Pandey, D., Varmola, M., & Del Lungo, A. (2002). Tropical forest plantation areas, 1995.
Pang, H., Chen, Y., He, J., Guo, D., Pan, X., Ma, Y., et al. (2020). Cation exchange resin-induced hydrolysis for improving biodegradability of waste activated sludge: Characterization of dissolved organic matters and microbial community. Bioresource Technology, 302, 122870.
Pavlović, M. D., Buntić, A. V., Mihajlovski, K. R., Šiler-Marinković, S. S., Antonović, D. G., Radovanović, Ž, & Dimitrijević-Branković, S. I. (2014). Rapid cationic dye adsorption on polyphenol-extracted coffee grounds—A response surface methodology approach. Journal of the Taiwan Institute of Chemical Engineers, 45(4), 1691–1699.
Pergent, G., Rico-Raimondino, V., & Pergent-Martini, C. (1997). Fate of primary production in Posidonia oceanica meadows of the Mediterranean. Aquatic Botany, 59(3–4), 307–321.
Pinos-Rodríguez, J. M., Zamudio, M., & González, S. S. (2008). The effect of plant age on the chemical composition of fresh and ensiled Agave salmiana leaves. South African Journal of Animal Science, 38(1), 43–50.
Quiroz, D. C., Quintanilla, J. Á. V., & Pineda, A. C. (2007). El género Agave L. bajo cultivo: Taxonomía, distribución y usos. Revista Mexicana de Ciencias Forestales, 32(101), 57–70.
Radhika, M., & Palanivelu, K. (2006). Adsorptive removal of chlorophenols from aqueous solution by low cost adsorbent—Kinetics and isotherm analysis. Journal of Hazardous Materials, 138(1), 116–124.
Rangabhashiyam, S., Nakkeeran, E., Anu, N., & Selvaraju, N. (2015). Biosorption potential of a novel powder, prepared from Ficus auriculata leaves, for sequestration of hexavalent chromium from aqueous solutions. Research on Chemical Intermediates, 41(11), 8405–8424.
Rashid, R. A., Jawad, A. H., Ishak, M. A. M., & Kasim, N. N. (2016). KOH-activated carbon developed from biomass waste: Adsorption equilibrium, kinetic and thermodynamic studies for Methylene blue uptake. Desalination and Water Treatment, 57(56), 27226–27236.
Rojas, G. A., Solano, J. P. L., & Pérez, J. E. R. (2007). Diversidad genética en poblaciones de agaves pulqueros (Agave spp.) del nororiente del Estado de México. Revista Fitotecnia Mexicana, 30(1), 1–12.
Saha, S. C., Das, B. K., Ray, P. K., Pandey, S. N., & Goswami, K. (1991). Infrared spectra of raw and chemically modified pineapple leaf fiber (Annanus comosus). Journal of Applied Polymer Science, 43(10), 1885–1890.
Salman, J. M., Njoku, V. O., & Hameed, B. H. (2011). Batch and fixed-bed adsorption of 2, 4-dichlorophenoxyacetic acid onto oil palm frond activated carbon. Chemical Engineering Journal, 174(1), 33–40.
Sánchez-Machado, D. I., López-Cervantes, J., Sendón, R., & Sanches-Silva, A. (2017). Aloe vera: Ancient knowledge with new frontiers. Trends in Food Science & Technology, 61, 94–102.
Şenol, Z. M., Messaoudi, N. El, Fernine, Y., & Keskin, Z. S. (2023). Bioremoval of rhodamine B dye from aqueous solution by using agricultural solid waste (almond shell): experimental and DFT modeling studies. Biomass Conversion and Biorefinery, 1–14.
Shariati-Rad, M., Irandoust, M., Amri, S., Feyzi, M., & Ja’fari, F. (2014). Magnetic solid phase adsorption, preconcentration and determination of methyl orange in water samples using silica coated magnetic nanoparticles and central composite design. International Nano Letters, 4(4), 91–101.
Shawabkeh, R. A., & Tutunji, M. F. (2003). Experimental study and modeling of basic dye sorption by diatomaceous clay. Applied Clay Science, 24(1–2), 111–120.
Shi, Y., Ge, Y., Chang, J., Shao, H., & Tang, Y. (2013). Garden waste biomass for renewable and sustainable energy production in China: Potential, challenges and development. Renewable and Sustainable Energy Reviews, 22, 432–437.
Silva, H., Sagardia, S., Seguel, O., Torres, C., Tapia, C., Franck, N., & Cardemil, L. (2010). Effect of water availability on growth and water use efficiency for biomass and gel production in Aloe Vera (Aloe barbadensis M.). Industrial Crops and Products, 31(1), 20–27.
Singh, P., Vishnu, M. C., Sharma, K. K., Singh, R., Madhav, S., Tiwary, D., & Mishra, P. K. (2016). Comparative study of dye degradation using TiO2-activated carbon nanocomposites as catalysts in photocatalytic, sonocatalytic, and photosonocatalytic reactor. Desalination and Water Treatment, 57(43), 20552–20564.
Son, Y. K., Lee, M. H., & Han, Y. N. (2005). A new antipsychotic effective neolignan fromfirmiana simplex. Archives of Pharmacal Research, 28(1), 34–38.
Spring, A., Diro, M., A Brandt, S., Tabogie, E., Wolde-Michael, G., McCabe, J. T., et al. (1997). Tree against hunger: enset-based agricultural systems in Ethiopia. American Association for the Advancement of science.
Sulyman, M., Namiesnik, J., & Gierak, A. (2017). Low-cost adsorbents derived from agricultural by-products/wastes for enhancing contaminant uptakes from wastewater: a review. Polish Journal of Environmental Studies, 26(3), 497–510.
Sundararaman, B., & Muthuramu, K. L. (2016). A comparison of mango seed kernel powder, mango leaf powder and Manilkara zapota seed powder for decolorization of methylene blue dye and antimicrobial activity. Journal of Environmental Biology, 37(6), 1315.
Swamy, M. M., Nagabhushana, B. M., Krishna, R. H., Kottam, N., Raveendra, R. S., & Prashanth, P. A. (2017). Fast adsorptive removal of methylene blue dye from aqueous solution onto a wild carrot flower activated carbon: Isotherms and kinetics studies. Water Treat, 71, 399–405.
Tahir, T. A., & Hamid, F. S. (2012). Vermicomposting of two types of coconut wastes employing Eudrilus eugeniae: A comparative study. International Journal of Recycling of Organic Waste in Agriculture, 1(1), 1–6.
Thang, N. H., Khang, D. S., Hai, T. D., Nga, D. T., & Tuan, P. D. (2021). Methylene blue adsorption mechanism of activated carbon synthesised from cashew nut shells. RSC Advances, 11(43), 26563–26570.
Tkaczyk, A., Mitrowska, K., & Posyniak, A. (2020). Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Science of the Total Environment, 717, 137222.
Tong, K. S., Kassim, M. J., & Azraa, A. (2011). Adsorption of copper ion from its aqueous solution by a novel biosorbent Uncaria gambir: Equilibrium, kinetics, and thermodynamic studies. Chemical Engineering Journal, 170(1), 145–153.
Tran, H. N., Nguyen, H. C., Woo, S. H., Nguyen, T. V., Vigneswaran, S., Hosseini-Bandegharaei, A., et al. (2019). Removal of various contaminants from water by renewable lignocellulose-derived biosorbents: A comprehensive and critical review. Critical Reviews in Environmental Science and Technology, 49(23), 2155–2219.
Tran, H. N., You, S.-J., Nguyen, T. V., & Chao, H.-P. (2017). Insight into the adsorption mechanism of cationic dye onto biosorbents derived from agricultural wastes. Chemical Engineering Communications, 204(9), 1020–1036.
Tsibranska, I., & Hristova, E. (2011). Comparison of different kinetic models for adsorption of heavy metals onto activated carbon from apricot stones. Bulgarian Chemical Communications, 43(3), 370–377.
Uçar, S., Erdem, M., Tay, T., & Karagöz, S. (2009). Preparation and characterization of activated carbon produced from pomegranate seeds by ZnCl2 activation. Applied Surface Science, 255(21), 8890–8896.
Van Thuan, T., Quynh, B. T. P., Nguyen, T. D., & Bach, L. G. (2017). Response surface methodology approach for optimization of Cu2+, Ni2+ and Pb2+ adsorption using KOH-activated carbon from banana peel. Surfaces and Interfaces, 6, 209–217.
Vedula, S. S., & Yadav, G. D. (2022). Wastewater treatment containing methylene blue dye as pollutant using adsorption by chitosan lignin membrane: Development of membrane, characterization and kinetics of adsorption. Journal of the Indian Chemical Society, 99(1), 100263.
Venegas-Gomez, A., Gomez-Corzo, M., Macias-Garcia, A., & Carrasco-Amador, J. P. (2020). Charcoal obtained from cherry stones in different carbonization atmospheres. Journal of Environmental Chemical Engineering, 8(2), 103561.
Wang, Y., Peng, C., Padilla-Ortega, E., Robledo-Cabrera, A., & López-Valdivieso, A. (2020). Cr (VI) adsorption on activated carbon: Mechanisms, modeling and limitations in water treatment. Journal of Environmental Chemical Engineering, 8(4), 104031.
Wazir, A. H., Waseem, I., Qureshi, I., & Manan, A. (2020). Saccharum Arundinaceum leaves as a versatile biosorbent for removal of methylene blue dye from wastewater. Environmental Engineering Science, 37(11), 737–745.
Weng, C.-H., Lin, Y.-T., & Tzeng, T.-W. (2009). Removal of methylene blue from aqueous solution by adsorption onto pineapple leaf powder. Journal of Hazardous Materials, 170(1), 417–424.
Williamson, G., Dionisi, F., & Renouf, M. (2011). Flavanols from green tea and phenolic acids from coffee: Critical quantitative evaluation of the pharmacokinetic data in humans after consumption of single doses of beverages. Molecular Nutrition & Food Research, 55(6), 864–873.
Xiong, L., Yang, Y., Mai, J., Sun, W., Zhang, C., Wei, D., et al. (2010). Adsorption behavior of methylene blue onto titanate nanotubes. Chemical Engineering Journal, 156(2), 313–320.
Xue, X., He, X., & Zhao, Y. (2012). Adsorptive properties of acid-heat activated rectorite for Rhodamine B removal: Equilibrium, kinetic studies. Desalination and Water Treatment, 37(1–3), 259–267.
Yakout, S. M., Daifullah, A. A. M., & El-Reefy, S. A. (2013). Adsorption of naphthalene, phenanthrene and pyrene from aqueous solution using low-cost activated carbon derived from agricultural wastes. Adsorption Science & Technology, 31(4), 293–302.
Yeow, P. K., Wong, S. W., & Hadibarata, T. (2021). Removal of azo and anthraquinone dye by plant biomass as adsorbent—a review. Biointerface Research in Applied Chemistry, 11(1), 8218–8232.
Yimer, J., Yadav, O. P., Kebede, T., & Mohammed, J. (2014). Kinetics and equilibrium study of adsorption of phenol red on teff (Eragrostis teff) husk activated carbon. Int J Innov Sci Res, 11, 471–476.
Yongram, C., Sungthong, B., Puthongking, P., & Weerapreeyakul, N. (2019). Chemical composition, antioxidant and cytotoxicity activities of leaves, bark, twigs and oleo-resin of Dipterocarpus alatus. Molecules, 24(17), 3083.
Yorgun, S., & Yıldız, D. (2015). Preparation and characterization of activated carbons from Paulownia wood by chemical activation with H3PO4. Journal of the Taiwan Institute of Chemical Engineers, 53, 122–131.
Zeydouni, G., Couto, S. R., Nourmoradi, H., Basiri, H., Amoatey, P., Esmaeili, S., et al. (2018). H2SO4-modified Aloe vera leaf shells for the removal of P-chlorophenol and methylene blue from aqueous environment. Toxin Reviews, 89(1), 57–67.
Zhou, Y., Zhang, L., & Cheng, Z. (2015). Removal of organic pollutants from aqueous solution using agricultural wastes: A review. Journal of Molecular Liquids, 212, 739–762.
Acknowledgements
Authors are very much thankful all staff at University of Babylon, Iraq for Financial support and all facilities to achieve this project.
Author information
Authors and Affiliations
Contributions
Fouad Fadhil Al-Qaim collected the related papers and wrote the first draft. Zainab Haider Mussa revised the final draft before submission. Lubna Raad Al-Ameer collected the references and improved the final revised draft. Issa Farhan Deyab revised the whole manuscript and improved the structure of the final draft. Hesam Kamyab revised the whole manuscript and improvement the English language. Shreeshivadasan Chelliapan revised the whole manuscript and improvement the English language. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All authors have read, understood, and have complied as applicable with the statement on “ethical responsibilities of authors” as found in the instructions for authors.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mussa, Z.H., Al-Ameer, L.R., Al-Qaim, F.F. et al. A comprehensive review on adsorption of methylene blue dye using leaf waste as a bio-sorbent: isotherm adsorption, kinetics, and thermodynamics studies. Environ Monit Assess 195, 940 (2023). https://doi.org/10.1007/s10661-023-11432-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-11432-1