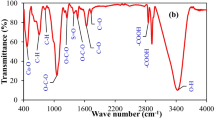
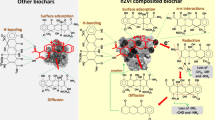
Abstract
The presence of endocrine-disrupting chemicals in municipal wastewater has emerged as a threat to human health and the environment. Therefore, this study aimed to develop biochar-cobalt ferrite (BCF) nanocomposite for the removal of methylparaben from water under the full factorial experimental design of 4 factors with 3 levels (34). The biochar-CoFe2O4 nanocomposite was developed by co-precipitation method from cobalt ferrite and biochar of Eucalyptus tree bark. Adsorbent surface morphology and functional and elemental composition were carried out by scanning electron microscopy (SEM), Fourier transform infrared (FTIR) spectroscopy, and energy-dispersive X-ray spectroscopy (EDS) techniques which showed the presence of cracks with a rough surface, reasonable surface chemical composition, and many chemical functional groups, respectively. The experimental and predicted adsorption efficiencies ranged from 25.3 to 85.6% and 21.8 to 80.3%, respectively. The maximum adsorption performance (85.6%) reduced the methylparaben concentration from 27.5 to 4.0 mg/L at the optimum condition of adsorbent dose of 55 mg/100 mL, pH 6, contact time 90 min, and the initial methylparaben concentration of 27.5 mg/L. However, the adsorbent dose was the most influential main factor whereas the least influential was the interaction between solution pH and contact time under the regression model. The model also showed that 69% methylparaben removal was described by the regression model. The experimental data best fitted with the Freundlich model indicate multilayer adsorption which is the implication of physisorption. The sorption mechanism is attributed to Vander Waals forces, H-bonding, and dipole interaction. This BCF nanocomposite adsorbent appears to be promising for the removal of methylparaben from wastewater, but a further optimization process is essential to boost the treatment performance.







Similar content being viewed by others
Availability of data and materials
All data are fully available without restriction.
References
Ahmaruzzaman, M. (2021). Biochar based nanocomposites for photocatalytic degradation of emerging organic pollutants from water and wastewater. Materials Research Bulletin, 140, 111262. https://doi.org/10.1016/j.materresbull.2021.111262
Ahmed, W., Mehmood, S., Núñez-Delgado, A., Ali, S., Qaswar, M., Khan, Z. H., et al. (2021). Utilization of Citrullus lanatus L. seeds to synthesize a novel MnFe2O4-biochar adsorbent for the removal of U(VI) from wastewater: Insights and comparison between modified and raw biochar. Science of the Total Environment, 771. https://doi.org/10.1016/j.scitotenv.2021.144955
Ajiboye, T. O., Oyewo, O. A., & Onwudiwe, D. C. (2021). Photocatalytic removal of parabens and halogenated products in wastewater : A review. Environmental Chemistry Letters, 19. Springer International Publishing. https://doi.org/10.1007/s10311-021-01263-2
Alaqarbeh, M., Khalili, F. I., & Kanoun, O. (2019). Manganese ferrite (MnFe24) as potential nanosorbent for adsorption of uranium (VI) and thorium (IV). Journal of Radioanalytical and Nuclear Chemistry, (VI). https://doi.org/10.1007/s10967-019-06953-4
Ángeles, M. D. L., Romero, B., Nuria, B., Botella, B., & Rico, D. P. (2019). Removal of emerging pollutants in water treatment plants : Adsorption of methyl and propylparaben onto powdered activated carbon. Adsorption, 25(5), 983–999. https://doi.org/10.1007/s10450-019-00120-7
Azizi, D., Arif, A., Blair, D., Dionne, J., Filion, Y., Ouarda, Y., et al. (2022). A comprehensive review on current technologies for removal of endocrine disrupting chemicals from wastewaters. Environmental Research, 207, 112196. https://doi.org/10.1016/j.envres.2021.112196
Bereketoglu, C., & Pradhan, A. (2019). Comparative transcriptional analysis of methylparaben and propylparaben in zebra fi sh. Science of the Total Environment, 671, 129–139. https://doi.org/10.1016/j.scitotenv.2019.03.358
Carmalin, S. A. (2018). Removal of emerging contaminants from the environment by adsorption. Ecotoxicology and Environmental Safety, 150, 1–17. https://doi.org/10.1016/j.ecoenv.2017.12.026
Chakhtouna, H., Benzeid, H., Zari, N., & Bouhfid, R. (2021). Functional CoFe2O4-modified biochar derived from banana pseudostem as an efficient adsorbent for the removal of amoxicillin from water. Separation and Purification Technology, 266, 118592. https://doi.org/10.1016/j.seppur.2021.118592
Chen, H., Chiou, C., Wu, Y., Chang, C., & Lai, Y. (2021). Magnetic nanoadsorbents derived from magnetite and graphene oxide for simultaneous adsorption of nickel ion, methylparaben, and reactive black 5. Desalination and Water Treatmen, 224, 168–177. https://doi.org/10.5004/dwt.2021.27114
Chen, J., Pycke, B. F. G., Brownawell, B. J., Kinney, C. A., Furlong, E. T., Kolpin, D. W., & Halden, R. U. (2017). Occurrence, temporal variation, and estrogenic burden of five parabens in sewage sludge collected across the United States. Science of the Total Environment, 593–594, 368–374. https://doi.org/10.1016/j.scitotenv.2017.03.162
Chen, L., Ma, R., Zhang, Z., Huang, M., Cai, C., Zhang, R., et al. (2019). Comprehensive investigation and comparison of surface microstructure of fractionated potato starches. Food Hydrocolloids, 89, 11–19. https://doi.org/10.1016/j.foodhyd.2018.10.017
Cheng, L., Luo, S., Li, X., Zhang, S., Nguyen, T. T., Guo, M., & Gao, X. (2021). Ultrasound-assisted heterogeneous Fenton-like process for methylene blue removal using magnetic MnFe2O4/biochar nanocomposite. Applied Surface Science, 566, 150654. https://doi.org/10.1016/j.apsusc.2021.150654
Cheng, N., Wang, B., Wu, P., Lee, X., Xing, Y., Chen, M., & Gao, B. (2021). Adsorption of emerging contaminants from water and wastewater by modified biochar: A review. Environmental Pollution, 273, 116448. https://doi.org/10.1016/j.envpol.2021.116448
Cuba-Chiem, L. T., Huynh, L., Ralston, J., & Beattie, D. A. (2008). In situ particle film ATR FTIR spectroscopy of carboxymethyl cellulose adsorption on talc: Binding mechanism, pH effects, and adsorption kinetics. Langmuir, 24(15), 8036–8044. https://doi.org/10.1021/la800490t
Dawood, S., Sen, T. K., & Phan, C. (2016). Adsorption removal of Methylene Blue ( MB ) dye from aqueous solution by bio-char prepared from Eucalyptus sheathiana bark : Kinetic, equilibrium, mechanism, thermodynamic and process design. Desalination and Water Treatment ISSN, 3994. https://doi.org/10.1080/19443994.2016.1188732
Dhangar, K., & Kumar, M. (2020). Tricks and tracks in removal of emerging contaminants from the wastewater through hybrid treatment systems: A review. Science of the Total Environment, 738(336), 140320. https://doi.org/10.1016/j.scitotenv.2020.140320
Du, M., Zhang, Y., Wang, Z., Lv, M., Tang, A., Yu, Y., et al. (2022). Insight into the synthesis and adsorption mechanism of adsorbents for efficient phosphate removal : Exploration from synthesis to modification. Chemical Engineering Journal, 442(P1), 136147. https://doi.org/10.1016/j.cej.2022.136147
Enaime, G., Baçaoui, A., Yaacoubi A., & Lübken, M. (2020). Biochar for wastewater treatment-conversion technologies and applications. Applied Sciences, 10. https://doi.org/10.3390/app10103492
Faria, J. L., Silva, C. G., Fernandes, R. A., Sampaio, M. J., & Draz, G. (2020). Efficient removal of parabens from real water matrices by a metal-free carbon nitride photocatalyst. Science of the Total Environment, 716. https://doi.org/10.1016/j.scitotenv.2019.135346
Fernandez-Sanroman, A., Acevedo-García, V., Pazos, M., Rosales, E., & Sanrom, M. A. (2020) Removal of sulfamethoxazole and methylparaben using hydrocolloid and fi ber industry wastes : Comparison with biochar and laccase-biocomposite. Journal of Cleaner Production Journal, 271. https://doi.org/10.1016/j.jclepro.2020.122436
Fito, J., Abrham, S., & Angassa, K. (2020). Adsorption of methylene blue from textile industrial wastewater onto activated carbon of parthenium hysterophorus. International Journal of Environmental Research, 14(5), 501–511. https://doi.org/10.1007/s41742-020-00273-2
Fito, J., Kefeni, K. K., & Nkambule, T. T. I. (2022). The potential of biochar-photocatalytic nanocomposites for removal of organic micropollutants from wastewater. Science of the Total Environment, 829, 154648. https://doi.org/10.1016/j.scitotenv.2022.154648
Fito, J., & Van Hulle, S. W. H. (2021). Wastewater reclamation and reuse potentials in agriculture: Towards environmental sustainability. Environment, Development and Sustainability, 23(3), 2949–2972. https://doi.org/10.1007/s10668-020-00732-y
Gamarra-güere, C. D., Dionisio, D., Roberto, M., Lanza, V., & Jesus, A. D. (2022). Application of Fenton, photo-Fenton and electro-Fenton processes for the methylparaben degradation : A comparative study. Journal of Environmental Chemical Engineering, 10, 106992. https://doi.org/10.1016/j.jece.2021.106992
Gan, L., Zhong, Q., Geng, A., Wang, L., Song, C., Han, S., et al. (2019). Cellulose derived carbon nanofiber: A promising biochar support to enhance the catalytic performance of CoFe2O4 in activating peroxymonosulfate for recycled dimethyl phthalate degradation. Science of the Total Environment, 694, 133705. https://doi.org/10.1016/j.scitotenv.2019.133705
Gao, M., Wang, W., Yang, H., & Ye, B. C. (2020). Efficient removal of fluoride from aqueous solutions using 3D flower-like hierarchical zinc-magnesium-aluminum ternary oxide microspheres. Chemical Engineering Journal, 380, 122459. https://doi.org/10.1016/j.cej.2019.122459
Ghorbani, F., Kamari, S., Askari, F., Molavi, H., & Fathi, S. (2021). Production of nZVI–Cl nanocomposite as a novel eco–friendly adsorbent for efficient As (V) ions removal from aqueous media: Adsorption modeling by response surface methodology. Sustainable Chemistry and Pharmacy, 21, 100437. https://doi.org/10.1016/j.scp.2021.100437
Huang, R., Yang, J., Cao, Y., Dionysiou, D. D., & Wang, C. (2022). Peroxymonosulfate catalytic degradation of persistent organic pollutants by engineered catalyst of self-doped iron/carbon nanocomposite derived from waste toner powder. Separation and Purification Technology, 291, 120963. https://doi.org/10.1016/j.seppur.2022.120963
Huang, Y., Chen, Q., Wang, Z., Yan, H., & Chen, C. (2021). Abatement technology of endocrine-disrupting chemicals ( EDCs ) by means of enhanced coagulation and ozonation for wastewater reuse. Chemosphere, 285, 131515. https://doi.org/10.1016/j.chemosphere.2021.131515
Iqbal, M. B. T. T., & Snobia, A. H. (2017). Wet chemical co-precipitation synthesis of nickel ferrite nanoparticles and their characterization. Journal of Inorganic and Organometallic Polymers and Materials, 27, 1430–1438. https://doi.org/10.1007/s10904-017-0598-5
Jiang, J., Zhou, Z., & Sharma, V. K. (2013). Occurrence, transportation, monitoring and treatment of emerging micro-pollutants in waste water—A review from global views. Microchemical Journal, 110, 292–300. https://doi.org/10.1016/j.microc.2013.04.014
Jiang, M., Chen, L., & Niu, N. (2022). Enhanced adsorption for malachite green by functionalized lignin magnetic composites: Optimization, performance and adsorption mechanism. Journal of Molecular Structure, 1260, 132842. https://doi.org/10.1016/j.molstruc.2022.132842
Kang, Z., Jia, X., Zhang, Y., Kang, X., Ge, M., Liu, D., et al. (2022). A review on application of biochar in the removal of pharmaceutical pollutants through adsorption and persulfate-based AOPs. Sustainability (Switzerland), 14(16). https://doi.org/10.3390/su141610128
Kannan, K. (2013). Parabens in sediment and sewage sludge from the United States, Japan, and Korea: Spatial distribution and temporal trends. Environmental Science & Technology, 47, 10895−10902. https://doi.org/10.1021/es402574k
Kashif, M., Kashif, A., Fuwad, A., & Choi, Y. (2021). Current advances in treatment technologies for removal of emerging contaminants from water—a critical review. Coordination Chemistry Reviews, 442, 213993. https://doi.org/10.1016/j.ccr.2021.213993
Kokkinos, P., & Mantzavinos, D. (2020). Current trends in the application of nanomaterials for the removal of emerging micropollutants and. molecules, 25, 1–31. https://doi.org/10.3390/molecules25092016
Li, A., Ge, W., Liu, L., & Qiu, G. (2022a). Preparation, adsorption performance and mechanism of MgO-loaded biochar in wastewater treatment : A review. Environmental Research, 212, 113341. https://doi.org/10.1016/j.envres.2022.113341
Li, J., Jiang, J., Yan, S., Sun, S., Wang, L., Zhou, Y., et al. (2019). Oxidation of methylparaben (MeP) and p - hydroxybenzoic acid (p -HBA) by manganese dioxide (MnO 2) and effects of iodide : Efficiency, products, and toxicity. Science of the Total Environment, 661, 670–677. https://doi.org/10.1016/j.scitotenv.2019.01.090
Li, J., Gou, G., Zhao, H., Liu, C., Li, N., Li, L., et al. (2022b). Efficient peroxymonosulfate activation by CoFe2O4-CeO2 composite: Performance and catalytic mechanism. Chemical Engineering Journal, 435(P1), 134840. https://doi.org/10.1016/j.cej.2022.134840
Li, X., Wang, H., Zhang, G., Zhou, T., & Wu, F. (2021). Hydrothermal synthesis of magnetic nano-CoFe2O4 catalyst and its enhanced degradation of amoxicillin by activated permonosulfate. Water Science and Technology, 84(12), 3616–3628. https://doi.org/10.2166/wst.2021.460
Liu, S., Li, X., Wang, D., Zhang, D. (2020) Investigations on the mechanism of the microstructural evolution of different coal ranks under liquid nitrogen cold soaking. Energy Sources, Part a: Recovery, Utilization and Environmental Effects, 1–17. https://doi.org/10.1080/15567036.2020.1841856
Liu, W., Song, X., Na, Z., Li, G., & Luo, W. (2022). Strategies to enhance micropollutant removal from wastewater by membrane bioreactors: Recent advances and future perspectives. Bioresource Technology, 344, 126322. https://doi.org/10.1016/j.biortech.2021.126322
Luo, D., Wang, L., Nan, H., Cao, Y., Wang, H., Thakur, et al. (2022). Phosphorus adsorption by functionalized biochar: A review. Environmental Chemistry Letters 2022, 1(0123456789), 1–28. https://doi.org/10.1007/s10311-022-01519-5
Mahouachi, L., Rastogi, T., Palm, W., Ghorbel-abid, I., Ben, D., Chehimi, H., & Kümmerer, K. (2020). Natural clay as a sorbent to remove pharmaceutical micropollutants from wastewater. Chemosphere, 258, 127213. https://doi.org/10.1016/j.chemosphere.2020.127213
Mao, H., Li, H., Li, Y., Li, L., Yin, L., & Yang, Z. (2020). Four typical personal care products in a municipal wastewater treatment plant in China : Occurrence, removal efficiency, mass loading and emission. Ecotoxicology and Environmental Safety, 188, 109818. https://doi.org/10.1016/j.ecoenv.2019.109818
Meng, G., Li, A., Yang, W., Liu, F., Yang, X., & Zhang, Q. (2007). Mechanism of oxidative reaction in the post crosslinking of hypercrosslinked polymers. European Polymer Journal, 43(6), 2732–2737. https://doi.org/10.1016/j.eurpolymj.2007.03.011
Mishra, V., Sureshkumar, M. K., Gupta, N., & Kaushik, C. P. (2017). Study on sorption characteristics of uranium onto biochar derived from eucalyptus wood. Water, Air, and Soil Pollution, 228(8). https://doi.org/10.1007/s11270-017-3480-8
Moges, A., Nkambule, T. T. I., & Fito, J. (2022). The application of GO-Fe 3 O 4 nanocomposite for chromium adsorption from tannery industry wastewater. Journal of Environmental Management, 305, 114369. https://doi.org/10.1016/j.jenvman.2021.114369
Moreno-Marenco, A. R., Giraldo, L., & Moreno-Piraján, J. C. (2019). Parabens adsorption onto activated carbon: Relation with chemical and structural properties. Molecules, 24(23). https://doi.org/10.3390/molecules24234313
Neolaka, Y. A. B., Lawa, Y., Naat, J. N., Riwu, A. A. P., Iqbal, M., Darmokoesoemo, H., & Kusuma, H. S. (2020). The adsorption of Cr (VI) from water samples using graphene oxide-magnetic (GO-Fe3O4) synthesized from natural cellulose-based graphite (kusambi wood or Schleichera oleosa): Study of kinetics, isotherms and thermodynamics. Journal of Materials Research and Technology, 9(3), 6544–6556. https://doi.org/10.1016/j.jmrt.2020.04.040
Nezhadali, A., Koushali, S. E., & Divsar, F. (2021). Synthesis of polypyrrole-chitosan magnetic nanocomposite for the removal of carbamazepine from wastewater: Adsorption isotherm and kinetic study. Journal of Environmental Chemical Engineering, 9(4), 105648. https://doi.org/10.1016/j.jece.2021.105648
Nure, J. F., Shibeshi, N. T., Asfaw, S. L., Audenaer, W., & Van Hulle, S. W. H. (2017). COD and colour removal from molasses spent wash using activated carbon produced from bagasse fly ash of matahara sugar factory, Oromiya region. Ethiopia. Water SA, 43(3), 470–479. https://doi.org/10.4314/wsa.v43i3.12
Othmani, A., John, J., Rajendran, H., Mansouri, A., Sillanpää, M., & Velayudhaperumal Chellam, P. (2021). Biochar and activated carbon derivatives of lignocellulosic fibers towards adsorptive removal of pollutants from aqueous systems: Critical study and future insight. Separation and Purification Technology, 274, 119062. https://doi.org/10.1016/j.seppur.2021.119062
Rasheed, T., Bilal, M., Nabeel, F., Adeel, M., & Iqbal, H. M. N. (2019). Environmentally-related contaminants of high concern: Potential sources and analytical modalities for detection, quantification, and treatment. Environment International, 122, 52–66. https://doi.org/10.1016/j.envint.2018.11.038
Rathi, B. S., & Kumar, P. S. (2021). Application of adsorption process for effective removal of emerging contaminants from water and wastewater. Environmental Pollution, 280, 116995. https://doi.org/10.1016/j.envpol.2021.116995
Ru, J., Wang, X., Wang, F., Cui, X., Du, X., & Lu, X. (2021). UiO series of metal-organic frameworks composites as advanced sorbents for the removal of heavy metal ions : Synthesis, applications and adsorption mechanism. Ecotoxicology and Environmental Safety, 208, 111577. https://doi.org/10.1016/j.ecoenv.2020.111577
Shen, J., Huang, G., An, C., Xin, X., Huang, C., & Rosendahl, S. (2018). Removal of Tetrabromobisphenol A by adsorption on pinecone-derived activated charcoals: Synchrotron FTIR, kinetics and surface functionality analyses. Bioresource Technology, 247, 812–820. https://doi.org/10.1016/j.biortech.2017.09.177
Sheng, J., Xu, J., Qin, B., & Jiang, H. (2022). Three-dimensional flower-like magnetic CoFe-LDHs/CoFe2O4 composites activating peroxymonosulfate for high efficient degradation of aniline. Journal of Environmental Management, 310, 114693. https://doi.org/10.1016/j.jenvman.2022.114693
Sher, F., Hanif, K., Rafey, A., Khalid, U., Zafar, A., Ameen, M., & Lima, E. C. (2021). Removal of micropollutants from municipal wastewater using different types of activated carbons. Journal of Environmental Management, 278, 111302. https://doi.org/10.1016/j.jenvman.2020.111302
Sirajudheen, P., Nikitha, M. R., Karthikeyan, P., & Meenakshi, S. (2020). Perceptive removal of toxic azo dyes from water using magnetic Fe3O4 reinforced graphene oxide–carboxymethyl cellulose recyclable composite: Adsorption investigation of parametric studies and their mechanisms. Surfaces and Interfaces, 21(August), 100648. https://doi.org/10.1016/j.surfin.2020.100648
Smitha, B., Sridhar, S., & Khan, A. A. (2005). Chitosan-sodium alginate polyion complexes as fuel cell membranes. European Polymer Journal, 41(8), 1859–1866. https://doi.org/10.1016/j.eurpolymj.2005.02.018
Su, Z., Li, Q., Liu, Y., Hu, G. H., & Wu, C. (2009). Compatibility and phase structure of binary blends of poly(lactic acid) and glycidyl methacrylate grafted poly(ethylene octane). European Polymer Journal, 45(8), 2428–2433. https://doi.org/10.1016/j.eurpolymj.2009.04.028
Süsser, M., Nirschl, H., Morsch, P., Fuchs, S., & Linda, M. (2021a). Elimination of micropollutants from municipal wastewater by adsorption on powdered activated carbon and separation by innovative precoat filtration Part 1 : Adsorption capacity of activated carbons and initial filtration investigations. Separation and Purification Technology, 277. https://doi.org/10.1016/j.seppur.2021.119052
Süsser, M., Nirschl, H., Morsch, P., & Linda, M. (2021b). Elimination of micropollutants from municipal wastewater by adsorption on powdered activated carbon and separation by innovative precoat filtration Part 2 : Case study on the filtration of activated carbon using a cellulose precoat. Separation and Purification Technolog, 277(August). https://doi.org/10.1016/j.seppur.2021.119444
Tang, Q., Wang, F., Tang, M., Liang, J., & Ren, C. (2012). Study on pore distribution and formation rule of sepiolite mineral nanomaterials. Journal of Nanomaterials, 2012, 1–6. https://doi.org/10.1155/2012/382603
Thomaidis, N. S., Asimakopoulos, A. G., & Bletsou, A. A. (2012). Emerging contaminants: A tutorial mini-review. Global Nest Journal, 14(1), 72–79. https://doi.org/10.30955/gnj.000823
Tong, Y., Mcnamara, P. J., & Mayer, B. K. (2019) Adsorption of organic micropollutants onto biochar: A review of relevant kinetics, mechanisms and equilibrium. Environmental Science Water Research & Technology, 5, 821–838. https://doi.org/10.1039/c8ew00938d
UN-Water. (2021). Valuing water, The United Nations World Water Development Report 2021, The United Nations Educational, Scientific and Cultural Organization 7. Place de Fontenoy, 75352 Paris 07 SP, (Vol. 191), France.
UN-Water. (2020). Water and Climate Change, The United Nations World Water Development Report 2020, the United Nations Educational, Scientific and Cultural Organization 7. Place de Fontenoy 75352 Paris 07 SP. France. https://doi.org/10.1002/9781118786352.wbieg0793.pub2
Vela, N., Calín, M., Yáñez-gascón, M. J., Garrido, I., Pérez-lucas, G., & Fenoll, J. (2018). Photocatalytic oxidation of six endocrine disruptor chemicals in wastewater using ZnO at pilot plant scale under natural sunlight. Environmental Science and Pollution Research, 25, 34995–35007. https://doi.org/10.1007/s11356-018-1716-9
Vikrant, K., Kim, K. H., Ok, Y. S., Tsang, D. C. W., Tsang, Y. F., Giri, B. S., & Singh, R. S. (2018). Engineered/designer biochar for the removal of phosphate in water and wastewater. Science of the Total Environment, 616–617, 1242–1260. https://doi.org/10.1016/j.scitotenv.2017.10.193
Wang, C., Huang, R., Sun, R., Yang, J., & Sillanpää, M. (2021). A review on persulfates activation by functional biochar for organic contaminants removal: Synthesis, characterizations, radical determination, and mechanism. Journal of Environmental Chemical Engineering, 9(5). https://doi.org/10.1016/j.jece.2021.106267
Wang, C., Luo, D., Zhang, X., Huang, R., Cao, Y., Liu, G., et al. (2022a). Biochar-based slow-release of fertilizers for sustainable agriculture: A mini review. Environmental Science and Ecotechnology, 10, 100167. https://doi.org/10.1016/j.ese.2022.100167
Wang, C., Wang, H., & Gu, G. (2018). Ultrasound-assisted xanthation of cellulose from lignocellulosic biomass optimized by response surface methodology for Pb(II) sorption. Carbohydrate Polymers, 182, 21–28. https://doi.org/10.1016/j.carbpol.2017.11.004
Wang, G., Hambly, A. C., Dou, Y., Wang, G., Tang, K., & Andersen, H. R. (2022b). Polishing micropollutants in municipal wastewater, using biogenic manganese oxides in a moving bed biofilm reactor (BioMn-MBBR). Journal of Hazardous Materials, 427, 127889. https://doi.org/10.1016/j.jhazmat.2021.127889
Xu, Z., Xiang, Y., Zhou, H., Yang, J., He, Y., Zhu, Z., & Zhou, Y. (2021). Manganese ferrite modified biochar from vinasse for enhanced adsorption of levofloxacin: Effects and mechanisms. Environmental Pollution, 272, 115968. https://doi.org/10.1016/j.envpol.2020.115968
Yao, B., Luo, Z., Du, S., Yang, J., Zhi, D., & Zhou, Y. (2021). Sustainable biochar/MgFe2O4 adsorbent for levofloxacin removal: Adsorption performances and mechanisms. Bioresource Technology, 340, 125698. https://doi.org/10.1016/j.biortech.2021.125698
You, Y., Shi, Z., Li, Y., Zhao, Z., He, B., & Cheng, X. (2021). Magnetic cobalt ferrite biochar composite as peroxymonosulfate activator for removal of lomefloxacin hydrochloride. Separation and Purification Technology, 272. https://doi.org/10.1016/j.seppur.2021.118889
Zhang, H., Nengzi, L. C., Liu, Y., Gao, Y., & Cheng, X. (2020). Efficient removal of organic pollutant by activation of persulfate with magnetic Co3O4/CoFe2O4 composite. Arabian Journal of Chemistry, 13(5), 5332–5344. https://doi.org/10.1016/j.arabjc.2020.03.012
Zhao, T., Ma, X., Cai, H., Ma, Z., & Liang, H. (2020). Study on the adsorption of CuFe2O4-loaded corncob biochar for Pb (II). Molecules, 25(15). https://doi.org/10.3390/molecules25153456
Acknowledgements
We would like to thank the University of South Africa, College of Science, Engineering and Technology, and the Institute for Nanotechnology and Water Sustainability, Florida Science Campus, 1710, Johannesburg, South Africa for laboratory facilities.
Funding
This research work was supported by the University of South Africa (UNISA), the Institute for Nanotechnology and Water Sustainability.
Author information
Authors and Affiliations
Contributions
Authors Jemal Fito and Thabo T.I Nkambule contribute to the development of the research concept and preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fito, J., Nkambule, T.T.I. Synthesis of biochar-CoFe2O4 nanocomposite for adsorption of methylparaben from wastewater under full factorial experimental design. Environ Monit Assess 195, 241 (2023). https://doi.org/10.1007/s10661-022-10819-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-022-10819-w