Abstract
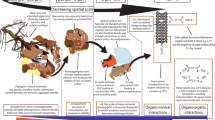
Shale-derived soils have higher clay, organic matter, and secondary Fe oxide content than other bedrock types, all of which can sequester Hg. However, shales also can be Hg-rich due to their marine formation. The objectives of this study were to determine the concentration and phase partitioning of Hg in seven upland weathering profiles from New York to Tennessee USA and use geochemical normalization techniques to estimate the extent of Hg inheritance from weathering of shale bedrock or sequestration of atmospheric Hg. Total Hg concentrations in unweathered shale ranged from 3 to 94 ng/g. Total Hg concentrations decreased with depth in the Ultisols and Alfisols, with total Hg concentrations ranging from 18 to 265 ng/g. Across all shale soils and rocks, the oxidizable fraction of Hg (15% H2O2 extraction) comprised a large portion of the total Hg at 68% ± 8%. This fraction was dominated by organic matter as confirmed with positive correlations between Hg and %LOI, but could also be impacted by Hg sulfides. Across all sites, the reducible fraction of Hg (citrate-bicarbonate-dithionite extraction) was only 10% ± 4% of the total Hg on average. Thus, secondary Fe oxides did not contain a significant portion of Hg, as commonly observed in tropical soils. Although colder sites had a higher organic matter and sequestered more Hg, τ values for Hg indexed to Ti suggest that atmospheric deposition, such as pollution sources in Ohio River Valley, drove the highest enrichment of Hg along the transect. These results demonstrate that shale-derived soils have a net accumulation and retention of atmospheric Hg, primarily through stabilization by organic matter.

© ESRI


Similar content being viewed by others
Data availability
Mercury concentration data are available in Supplemental Information.
Code availability
Software used to analyze the data is available in MATLAB version 2016 and Microsoft Excel 2010.
References
Adriano, D. C. (2001). Mercury. In Trace elements in terrestrial environments. Springer.
Amos, H. M., Jacob, D. J., Streets, D. G., & Sunderland, E. M. (2013). Legacy impacts of all-time anthropogenic emissions on the global mercury cycle. Global Biogeochemical Cycles, 27, 410–421.
Anderson, S. P., Dietrich, W. E., & Brimhall, G. H., Jr. (2002). Weathering profiles, mass-balance analysis, and rates of solute loss: Linkages between weathering and erosion in a small, steep catchment. Geological Society of America Bulletin, 114(9), 1143–1158.
Biester, H., Gosar, M., & Covelli, S. (2000). Mercury speciation in sediments affected by dumped mining residues in the drainage area of the Idrija mercury mine, Slovenia. Environmental Science & Technology, 34(16), 3330–3336.
Brantley, S. L., & Lebedeva, M. (2011). Learning to read the chemistry of regolith to understand the critical zone. Annual Review of Earth and Planetary Sciences, 39, 387–416.
Brimhall, G. H., & Dietrich, W. E. (1987). Constitutive mass balance relations between chemical composition, volume, density, porosity, and strain in metasomatic hydrochemical systems: Results on weathering and pedogenesis. Geochimica Et Cosmochimica Acta, 51, 567–587.
Buss, H. L., Lara, M. C., Moore, O. W., Kurtz, A. C., Schulz, M. S., & White, A. F. (2017). Lithological influences on contemporary and long-term regolith weathering at the Luquillo critical zone observatory. Geochimica Et Cosmochimica Acta, 196, 224–251.
Driscoll, C. T., Mason, R. P., Chan, H. M., Jacob, D. J., & Pirrone, N. (2013). Mercury as a global pollutant: Sources, pathways, and effects. Environmental Science & Technology, 47, 4967–4983.
Fiorentino, J. C., Enzweiler, J., & Angélica, R. S. (2011). Geochemistry of mercury along a soil profile compared to other elements and to the parental rock: Evidence of external input. Water, Air, & Soil Pollution, 221, 63–75.
Fu, X., Wang, J., Tan, F., Feng, X., Zeng, S., Chen, W., & Wang, D. (2015). Minerals and potentially hazardous trace elements in marine oil shale: New insights from the Shengli River North surface mine, northern Tibet, China. Environmental Earth Sciences, 73(7), 3137–3157.
Gabriel, M. C., & Williamson, D. G. (2004). Principal biogeochemical factors affecting the speciation and transport of mercury through the terrestrial environment. Environmental Geochemistry and Health, 26, 421–434.
Gee, G. W., Bauder, J. W., & Klute, A. (1986). Methods of soil analysis, part 1, physical and mineralogical methods. Soil Science Society of America.
Grimaldi, C., Grimaldi, M., & Guedron, S. (2008). Mercury distribution in tropical soil profiles related to origin of mercury and soil processes. Science of the Total Environment, 401, 121–129.
Guedron, S., Grimaldi, C., Chauvel, C., Spadini, L., & Grimaldi, M. (2006). Weathering versus atmospheric contributions to mercury concentrations in French Guiana soils. Applied Geochemistry, 21, 2010–2022.
Hojdová, M., Rohovec, J., Chrastný, V., Penížek, V., & Navrátil, T. (2015). The influence of sample drying procedures on mercury concentrations analyzed in soils. Bulletin of Environmental Contamination and Toxicology, 94, 570–576.
Holmes, P., James, K. A. F., & Levy, L. S. (2009). Is low-level environmental mercury exposure of concern to human health? Science of the Total Environment, 408, 171–182.
Hosterman, J.W. and Whitlow, S.T., 1981. Clay mineralogy of Devonian shales in the Appalachian Basin. Geol. Surv. Open-File Rep. (US);(United States), 81.
Hylander, L. D., & Meili, M. (2003). 500 years of mercury production: Global annual inventory by region until 2000 and associated emissions. Science of the Total Environment, 304, 13–27.
Issaro, N., Abi-Ghanem, C., & Bermond, A. (2009). Fractionation studies of mercury in soils and sediments: A review of the chemical reagents used for mercury extraction. Analytica Chimica Acta, 631(1), 1–12.
Keeler, G. J., Landis, M. S., Norris, G. A., Christianson, E. M., & Dvonch, J. T. (2006). Sources of mercury wet deposition in eastern Ohio, USA. Environmental Science & Technology, 40, 5874–5881.
Lamborg, C. H., Fitzgerald, W. F., O’Donnell, J., & Torgersen, T. (2002). A non-steady-state compartmental model of global-scale mercury biogeochemistry with interhemispheric atmospheric gradients. Geochimica Et Cosmochimica Acta, 66, 1105–1118.
Luo, Y., Duan, L., Wang, L., Xu, G., Wang, S., & Hao, J. (2014). Mercury concentrations in forest soils and stream waters in northeast and south China. Science of the Total Environment, 496, 714–720.
Martín, J. A. R., & Nanos, N. (2016). Soil as an archive of coal-fired power plant mercury deposition. Journal of Hazardous Materials, 308, 131–138.
Mehra, O. P., & Jackson, M. L. (2013). Iron oxide removal from soils and clays by a dithionite–citrate system buffered with sodium bicarbonate. In Clays and clay minerals (pp. 317–327). Cham: Pergamon.
National Oceanographic and Atmospheric Administration (NOAA) (2011) Climate Data Online: Monthly Observational Data. U.S. Department of Commerce, National Oceanic and Atmospheric Administration, National Environmental Satellite Data and Information Service, National Climatic Data Center. http://www.ncdc.noaa.gov/cdo-web/.
Nriagu, J., & Becker, C. (2003). Volcanic emissions of mercury to the atmosphere: Global and regional inventories. Science of the Total Environment, 304, 3–12.
Obrist, D., Johnson, D. W., & Lindberg, S. E. (2009). Mercury concentrations and pools in four Sierra Nevada forest sites, and relationships to organic carbon and nitrogen. Biogeosciences, 6, 765–777.
Obrist, D., Johnson, D. W., Lindberg, S. E., Luo, Y., Hararuk, O., Bracho, R., Battles, J. J., Dail, D. B., Edmons, R. L., Monson, R. K., Ollinger, S. V., Pallardy, S. G., Pregitzer, K. S., & Todd, D. E. (2011). Mercury distribution across 14 U.S. forests. Part 1: Spatial patterns of concentrations in biomass, litter, and soils. Environmental Science and Technology, 45, 3974–3981.
Richardson, J. B., & Friedland, A. J. (2015). Mercury in coniferous and deciduous upland forests in northern New England, USA: Implications of climate change. Biogeosciences, 12, 6737–6749.
Richardson, J. B., & Moore, L. (2020). A tale of three cities: Mercury in urban deciduous foliage and soils across land-uses in Poughkeepsie NY, Hartford CT, and Springfield MA USA. Science of The Total Environment, 715, 136869.
Richardson, J. B., Friedland, A. J., Engerbretson, T. R., Kaste, J. M., & Jackson, B. P. (2013). Spatial and vertical distribution of mercury in upland forest soils across the northeastern United States. Environmental Pollution, 182, 127–134.
Richardson, J. B., Aguirre, A. A., Buss, H. L., Toby O’Geen, A., Gu, X., Rempe, D. M., & Richter, D. D. B. (2018). Mercury sourcing and sequestration in weathering profiles at six critical zone observatories. Global Biogeochemical Cycles, 32(10), 1542–1555.
Risch, M. R., Gay, D. A., Fowler, K. K., Keeler, G. J., Backus, S. M., Blanchard, P., Barres, J. A., & Dvonch, J. T. (2012). Spatial patterns and temporal trends in mercury concentrations, precipitation depths, and mercury wet deposition in the North American Great Lakes region, 2002–2008. Environmental Pollution, 161, 261–271.
Roulet, M., Lucotte, M., Saint-Aubin, A., Tran, S., Rheault, I., Farella, N., Dezencourt, J., Passos, C. J. S., Soares, G. S., Guimaraes, J. R., & Mergler, D. (1998). The geochemistry of mercury in central Amazonian soils developed on the Alter-do-Chao formation of the lower Tapajos River Valley, Para state, Brazil. Science of the Total Environment, 223, 1–24.
Schuster, P. F., Schaefer, K. M., Aiken, G. R., Antweiler, R. C., Dewild, J. F., Gryziec, J. D., Gusmeroli, A., Hugelius, G., Jafarov, E., Krabbenhoft, D. P., & Liu, L. (2018). Permafrost stores a globally significant amount of mercury. Geophysical Research Letters, 45, 1463–1471.
Schwesig, D., & Matzner, E. (2001). Dynamics of mercury and methylmercury in forest floor and runoff of a forested watershed in Central Europe. Biogeochemistry, 53, 181–200.
Shi, J. B., Meng, M., Shao, J. J., Zhang, K. G., Zhang, Q. H., & Jiang, G. B. (2013). Spatial distribution of mercury in topsoil from five regions of China. Environmental Science and Pollution Research, 20, 1756–1761.
Skyllberg, U., Xia, K., Bloom, P. R., Nater, E. A., & Bleam, W. F. (2000). Binding of mercury (II) to reduced sulfur in soil organic matter along upland-peat soil transects. Journal of Environmental Quality, 29(3), 855–865.
Streets, D. G., Horowitz, H. M., Jacob, D. J., Lu, Z., Levin, L., Ter Schure, A. F., & Sunderland, E. M. (2017). Total mercury released to the environment by human activities. Environmental Science & Technology, 51, 5969–5977.
Streets, D. G., Horowitz, H. M., Lu, Z., Levin, L., Thackray, C. P., & Sunderland, E. M. (2019). Global and regional trends in mercury emissions and concentrations, 2010–2015. Atmospheric Environment, 201, 417–427.
Tsui, M. T. K., Blum, J. D., Finlay, J. C., Balogh, S. J., Nollet, Y. H., Palen, W. J., & Power, M. E. (2014). Variation in terrestrial and aquatic sources of methylmercury in stream predators as revealed by stable mercury isotopes. Environmental Science & Technology, 48, 10128–10135.
United States Energy Information Administration and Kuuskraa. V. 2011. World shale gas resources: an initial assessment of 14 regions outside the United States. US Department of Energy
Vaughan, W. J., & Russell, C. S. (2015). Freshwater recreational fishing: The national benefits of water pollution control. Routledge.
Xue, W., Kwon, S. Y., Grasby, S. E., Sunderland, E. M., Pan, X., Sun, R., Zhou, T., Yan, H., & Yin, R. (2019). Anthropogenic influences on mercury in Chinese soil and sediment revealed by relationships with total organic carbon. Environmental Pollution, 255, 113186.
Yu, X., Driscoll, C. T., Warby, R. A., Montesdeoca, M., & Johnson, C. E. (2014). Soil mercury and its response to atmospheric mercury deposition across the northeastern United States. Ecological Applications, 24, 812–822.
Zahir, F., Rizwi, S. J., Haq, S. K., & Khan, R. H. (2005). Low dose mercury toxicity and human health. Environmental Toxicology and Pharmacology, 20, 351–360.
Zhang, H., Li, Y., Luo, Y., & Christie, P. (2015). Anthropogenic mercury sequestration in different soil types on the southeast coast of China. Journal of Soils and Sediments, 15(4), 962–971.
Zhang, Y., Wang, M., Huang, B., Akhtar, M. S., Hu, W., & Xie, E. (2018). Soil mercury accumulation, spatial distribution and its source identification in an industrial area of the Yangtze Delta, China. Ecotoxicology and Environmental Safety, 163, 230–237.
Acknowledgements
Justin Richardson would like to thank Sumaya Hamdi and Corey Palmer for excavating and collecting the samples as well as Mark Butler, Ainsley McStay, and Eliza Fitzgerald for partial soil sample preparations.
Funding
This project was funded by the University of Massachusetts Amherst College of Natural Sciences with a grant to Justin Richardson.
Author information
Authors and Affiliations
Contributions
Justin Richardson designed the study, processed, and analyzed the samples, completed the statistical analyses and interpretations, and wrote the entire manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author states there are no conflicts of interests.
Consent to participate
No human subjects were used in this research.
Consent for publication
No human subjects were used in this research.
Human and animal rights
No animals were used in this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Richardson, J.B. Shale weathering profiles show Hg sequestration along a New York–Tennessee transect. Environ Geochem Health 44, 3515–3526 (2022). https://doi.org/10.1007/s10653-021-01110-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-021-01110-x