Abstract
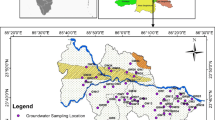
This study presents the groundwater quality assessment in the north of Isfahan, Iran. In the study area, assessment and measurement of groundwater hydrochemical parameters such as pH, total dissolved solids (TDS), electrical conductivity (EC), sodium absorption ratio (SAR), total hardness, major cations (K+, Na+, Ca2+ and Mg2+) and major anions (Cl−, \({\text{HCO}}_{ 3}^{ - } ,{\text{CO}}_{3}^{2 - }\) and \({\text{SO}}_{4}^{2 - }\)) concentrations were performed. Accordingly, the 66 water samples from different locations were collected during April and May 2015. Water samples collected in the field were analyzed in the laboratory for cations and anions using the standard methods. In this research, the analytical results of physiochemical parameters of groundwater were compared with the standard guideline values as recommended by the world health organization (WHO) for drinking and public health purposes. The pH values of groundwater samples varied from 7.05 to 8.95 with a mean of 7.78, indicating a neutral to slightly alkaline water. TDS values showed that 14% of the samples exceeds the desirable limit given by WHO. EC values varied from 213 to 4320 µS/cm, while 23% of the samples were more than the standard limit. Gibbs diagram had shown that 90% of the samples in the study area fall in the rock weathering zone, and this means that chemical weathering of rock-forming minerals is the main factor controlling the water chemistry in the study area. Irrigation suitability and risk assessment of groundwater are evaluated by measuring EC, %Na, SAR and RSC. According to the dominant cations and anions, five types of water were identified in the water samples: Ca-HCO3, Ca-SO4, Na-Cl, Na-HCO3 and Na-SO4. The results show that the majority of samples (30 samples, 45%) belongs to the mixed Na-SO4 water type. Correlation analysis and principal component analysis was used to identify the relationship between ions and physicochemical parameters. Results indicated that 18 stations of the study area had the best quality and can be used for irrigation and drinking purposes in the future.
























Similar content being viewed by others
References
Adams, S., Titus, R., Pietersen, K., & Harris, G. (2001). Hydrochemical characteristics of aquifers near Sutherland in the Western Karoo, South Africa. Journal of Hydrology, 241(1–2), 91–103.
Aghazadeh, N., & Moghaddam, A. A. (2011). Investigation of hydrochemical characteristics of groundwater in the Harzandat aquifer, Northwest of Iran. Environmental Monitoring and Assessment, 176, 183–195.
Ako, A. A., Shimada, J., Hosono, T., Kagabu, M., Ayuk, A. R., Nkeng, G. E., et al. (2012). Spring water quality and usability in the Mount Cameroon area revealed by hydrogeochemistry. Environmental Geochemistry and Health. doi:10.1007/s10653-012-9453-3.
Amidi, S. M., & Zahedi, M. (1988). Geology map of Kashan 1:250,000 sheets. Tehran: Geology Survey of Iran.
Babaahmadi, A., Safaei, H., Yassaghi, A., Vafa, H., Naeimi, A., Madanipour, S., et al. (2010). A study of Quaternary structures in the Qom region, West Central Iran. Journal of Geodynamics, 50, 355–367.
Baghvand, A., Nasrabadi, T., Bidhendi, N. G., Vosoogh, A., Karbassi, A., & Mehrdadi, N. (2010). Groundwater quality degradation of an aquifer in Iran central desert. Desalination, 260, 264–275.
Eaton, E. M. (1950). Significance of carbonate in irrigation water. Soil Science, 69, 12–133.
Evans, C. D., Davies, T. D., Wigington, P. J., Jr., Tranter, M., & Kretser, W. A. (1996). Use of factor analysis to investigate processes controlling the chemical composition of four streams in Adirondack Mountains, New York. Journal of Hydrology, 185, 297–316.
Ganyaglo, S. Y., Banoeng-Yakubo, B., Osae, S., Dampare, S. B., & Fianko, J. R. (2011). Water quality assessment of groundwater in some rock types in parts of the eastern region of Ghana. Environmental Earth Sciences, 62(5), 1055–1069.
Gibbs, R. J. (1970). Mechanisms controlling world water chemistry. Science, 17, 1088–1090.
Giridharan, L., Venugopal, T., & Jayaprakash, M. (2008). Evaluation of the seasonal variation on the geochemical parameters and quality assessment of the groundwater in the proximity of River Cooum, Chennai, India. Environmental Monitoring and Assessment, 143, 161–178.
Guey-Shin, S., Bai-You, C., Chi-Ting, C., Pei-Hsuan, Y., & TsunKuo, C. (2011). Applying factor analysis combined with Kriging and information entropy theory for mapping and evaluating the stability of groundwater quality variation in Taiwan. International Journal of Environmental Research and Public Health, 8, 1084–1109.
Guler, C., & Thyne, G. D. (2004). Hydrologic and geologic factors controlling surface and groundwater chemistry in Indian wells-Owens valley area, Southeastern California, USA. Journal of Hydrology, 285, 177–198.
Handa, B. K. (1969). Description and classification of media for hydro-geochemical investigations. Roorkee: Symposium on Ground Water Studies in Arid and Semiarid Regions.
Hem, J. D. (1991). Study and interpretation of the chemical characteristics of natural water. Book 2254 (3rd ed.). Jodhpur: Scientific Publishers.
Jalali, M. (2006). Chemical characteristics of groundwater in parts of mountainous region, Alvand, Hamedan, Iran. Environmental Geology, 51, 433–446.
Jeevanandam, M., Kannan, R., Srinivasalu, S., & Rammohan, V. (2007). Hydrogeochemistry and groundwater quality assessment of lower part of the Ponnaiyar River Basin, Cuddalore district, South India. Environmental Monitoring and Assessment, 132, 263–274.
Jiang, Y., & Yan, J. (2010). Effects of land use on hydrochemistry and contamination of Karst groundwater from Nandong underground river system, China. Water, Air, and Soil pollution, 210, 123–141.
Jolliffe, I. T. (2002). Principal component analysis (2nd ed., p. 487). New York: Springer.
Jordao, C. P., Ribeiro, P. R. S., Matos, A. T., Bastos, R. K. X., Fernandes, R. B. A., & Fontes, R. L. F. (2007). Environmental assessment of watercourses of the Turvo Limpo River basin at the Minas Gerais State, Brazil. Environmental Monitoring and Assessment, 127, 315–326.
Kaiser, H. F. (1958). The varimax criterion for analytic rotation in factor analysis. Psychometrika, 23, 187–200.
Kamtchueng, B. T., Fantong, W. Y., Ueda, A., Tiodjio, E. R., Anazawa, K., Wirmvem, M. J., et al. (2014). Assessment of shallow groundwater in Lake Nyos catchment (Cameroon, Central-Africa): Implications for hydrogeochemical controls and uses. Environment and Earth Science, 72, 3663–3678.
Kumar, M., Kumari, K., Ramanathan, A., & Saxena, R. (2007). A comparative evaluation of groundwater suitability for irrigation and drinking purposes in two intensively cultivated districts of Punjab, India. Journal of Environmental Geology, 53, 553–574.
Ludwig, F., Stober, I., & Bucher, K. (2011). Hydrochemical groundwater evolution in the bunter sandstone sequence of the Odenwald Mountain Range, Germany: A laboratory and field study. Aquatic Geochemistry, 17, 165–193.
Mano, J. K., Ghosh, S., & Padhy, P. K. (2013). Characterization and classification of hydrochemistry using multivariate graphical and hydrostatistical techniques. Research Journal Chemical Sciences, 3, 32–42.
Merkel, B., & Planer-Friedrich, B. (2008). Groundwater geochemistry—A practical guide to modeling of natural and contaminated aquatic systems. Berlin: Springer.
Meyback, M. (1987). Global chemical weathering of surficial rocks estimated from river dissolved loads. American Journal of Science, 287, 401–428.
Nagarajan, R., Rajmohan, N., Mahendran, U., & Senthamilkumar, S. (2010). Evaluation of groundwater quality and its suitability for drinking and agricultural use in Thanjavur city, Tamil Nadu, India. Environmental Monitoring and Assessment, 171, 289–308.
Piper, A. M. (1944). A graphic procedure in the geochemical interpretation of water-analyses. Eos, Transactions American Geophysical Union, 25(6), 914–928.
Raghunath, H. M. (2003). Groundwater. New Delhi: New Age International (P) Ltd.
Rao, N. S. (2002). Geochemistry of groundwater in parts of Guntur district, Andhra Pradesh, India. Environmental Geology, 41, 552–562.
Rao, S. V. L. (2003). Cluster analysis of groundwater quality data of Venkatagiri Taluq, Nellore district, Andhra Pradesh. Journal of the Geological Society of India, 62, 447–454.
Razo, I., Carrizales, L., Castro, J., Díaz-Barringa, F., & Monroy, M. (2004). Arsenic and heavy metal pollution of soil, water and sediments in a semi-arid climate mining area in Mexico. Water Air Soil Pollution, 152, 129–152.
Richards, L. A. (1954). Diagnosis and improvement of saline- alkali soils: Agriculture, Handbook 60 (vol. 160). Washington, DC: US Department of Agriculture.
Rowell, D. L. (1994). Soil science: Methods and applications. New York: Longman and Scientific Technical.
Schoeller, H. (1977). Qualitative evaluation of ground water resources (in methods and techniques of groundwater investigations and development), water resources series (vol. 33, pp. 44–52), UNESCO.
Srinivasamoorthy, K., Gopinath, M., Chidambaram, S., Vasanthavigar, M., & Sarma, V. S. (2014). Hydrochemical characterization and quality appraisal of groundwater from Pungar sub-basin, Tamilnadu, India. Journal of King Saud University-Science, 26, 37–52.
Subramani, T., Rajmohan, N., & Elango, L. (2010). Groundwater geochemistry and identification of hydrogeochemical processes in hard rock region, Southern India. Environmental Monitoring and Assessment, 162, 123–137.
Tatawat, R. K., & Chandel, S. C. P. (2008). A hydrochemical profile for assessing the groundwater quality of Jaipur City. Environmental Monitoring and Assessment, 143, 337–343.
Todd, D. K., & Mays, L. W. (2005). Ground-water hydrology. New York: Wiley.
USSL (United States Salinity Laboratory). (1954). Diagnosis and improvement of saline and alkaline soils. Washington: US Department of Agriculture.
WHO. (2004). Guidelines for drinking water quality, vol. 1 recommendations (3rd ed.). Geneva: WHO.
Wilcox, L. V. (1955). Classification and use of irrigation water. USDA circular 969. US Department of Agriculture, Washington, DC, p. 19.
Acknowledgements
The authors would like to thank the Amirkabir University of Technology (Polytechnic Tehran) for supporting this research. The contribution of Adonis Fard Mousavi, Omid Mazaheri and Mohammad Parsa is appreciated. Funding was provided by Amirkabir University of Technology (Grant No. 95930).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rezaei, A., Hassani, H. Hydrogeochemistry study and groundwater quality assessment in the north of Isfahan, Iran. Environ Geochem Health 40, 583–608 (2018). https://doi.org/10.1007/s10653-017-0003-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-017-0003-x