Abstract
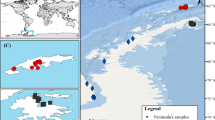
In this study, we used amplified fragment length polymorphism analysis to investigate the genetic structure of the terrestrial moss Pseudoscleropodium purum (Hedw.) M. Fleish. naturally exposed to different levels of atmospheric deposition of heavy metals. We also determined the heavy metal concentrations in samples of this moss to evaluate whether there was a relationship between atmospheric pollution and population genetic diversity. A low level of genetic diversity and a limited gene flow among populations were observed which is in accordance to the prevalence of asexual reproduction in this species. In addition, no significant correlation was found between metal content and gene diversity in P. purum, probably because of the common history of the sampled populations and/or to the lack of a drastic reduction of the size of the population; nonetheless, a clear genetic structure was evident in relation to the existing pollution gradient. Thus, based on the results of the principal coordinate analysis and Bayesian analysis of the genotypes, the mixed structure of the second most polluted population would suggest an ongoing differentiation of metal-tolerant genotypes in the most polluted sites of the sampling area.




Similar content being viewed by others
References
Bargagli R (1998) Trace elements in terrestrial plants. An ecophysiological approach to biomonitoring and biorecovery. Bargagli R. (ed) Springer, Berlin and Heidelberg, Germany; New York
Beerli P (2006) Comparison of Bayesian and maximum-likelihood inference of population genetic parameters. Bioinformatics 22:341–345
Boquete MT, Fernández JA, Carballeira A, Aboal JR (2013) Assessing the tolerance of terrestrial moss Pseudoscleropodium purum to high levels of atmospheric heavy metals: a reciprocal transplant study. Sci Tot Environ 461-462:552–559. doi:10.1016/j.scitotenv.2013.05.039
Briggs D (1972) Population differentiation in Marchantia polymorpha L. in various lead pollution levels. Nature 238:166–167. doi:10.1038/238166a0
Brown DH, House KL (1978) Evidence of a copper-tolerant ecotype of the hepatic Solenostoma crenulatum. Ann Bot-London 42:383–1392
Brown DH, Wells JM (1990) Physiological effects of heavy metals on the moss Rhytidiadelphus squarrosus. Ann Bot-London 66:641–647
Carballeira A, Couto JA, Fernández JA (2002) Estimation of background levels of various elements in terrestrial mosses from Galicia (NW of Spain). Water Air Soil Poll 133:235–252. doi:10.1023/A:1012928518633
Choudhury S, Panda SK (2005) Toxic effects, oxidative stress and ultrastructural changes in moss Taxithelium nepalense (Schwaegr) broth. under chromium and lead phytotoxicity. Water Air Soil Poll 167:73–90. doi:10.1007/s11270-005-8682-9
Crespo Pardo D, Terracciano S, Giordano S, Spagnuolo V (2014) Molecular markers based on PCR methods: a guideline for mosses. Cryptogam Briol 35(3):229–246. doi:10.7872/cryb.v35.iss3.2014.229
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Fernández CC, Shevock JR, Glazer AN, Thompson JN (2006) Cryptic species within the cosmopolitan desiccation-tolerant moss Grimia laevigata. Evolution 103:637–642. doi:10.1073/pnas.0510267103
Fernández JA, Aboal JR, Real C, Carballeira A (2007) A new moss biomonitoring method for detecting sources of small scale pollution. Atmos Environ 41:2098–2110. doi:10.1016/j.atmosenv.2006.10.072
Folkeson L (1979) Changes in the cover of mosses and lichens in coniferous woodland polluted with copper and zinc. In: Hytteborn H (ed) The use of ecological variables in environmental monitoring, Proceedings of the First Nordic Oikos Conference, 1978, Uppsala, Sweden, pp. 59–61
Frankham R, Ballou JD, Briscoe DA (2004) A primer of conservation genetics. Cambridge University Press, Cambridge, UK
Fritz S (2009) Vegetative reproduction and clonal diversity in pleurocarpous mosses (Bryophytina) of mesic habitats. A combined molecular and morpho-anatomical study in Pseudoscleropodium purum (Hedw.) M. Fleisch. ex Broth. (Brachytheciaceae), Pleurozium schreberi (Brid.) Mitt. (Hylocomiaceae) and Rhytidiadelphus squarrosus (Hedw.) Warnst. (Hylocomiaceae). Dissertation, Freien Universität, Berlin
Hoffmann AA, Willi Y (2008) Detecting genetic responses to environmental change. Nature Rev Genet 9:420–432. doi:10.1038/nrg2339
Hofman M (2007) The effect of genetic diversity on evolutionary potential. Local adaptation in the natterajck toad Bufo calamita in Sweden. Dissertation, Uppsala University
Huttunen S (2003) Reproduction of the mosses Pleurozium schreberi and Polia nutans in the surroundings of copper smelters at Harjavalta, S.W. Finland. J Bryol 25:41–47. doi:10.1179/037366803125002644
Jensen J.L., Bohonak A.J., Kelley S.T. (2005) Isolation by distance, web service. BMC Genetics 6: 13. V.3.23. http://ibdws.sdsu.edu/. Accessed 15 July 2015
Jules ES, Shaw AJ (1994) Adaptation to metal-contaminated soils in populations of the moss, Ceratodon purpureus: vegetative growth and reproductive expression. Am J Bot 81:791–797. doi:10.2307/2445660
Kimmerer RW (1991) Reproductive ecology of Tetraphis pellucida. II. Differential success of sexual and asexual propagules. Bryologist 94:284–288. doi:10.2307/3243966
Kimmerer RW (1994) Ecological consequences of sexual versus asexual reproduction in Dicranum flagellare and Tetraphis pellucida. Bryologist 97:20–25. doi:10.2307/3243344
King TJ (2003) Mosses and aspect; why is Scleropodium purum abundant on the north-facing sides of ant-hills? J Bryol 25:211–213. doi:10.1179/037366803235001689
Korpelainen H, von Cräutlein M, Kostamo K, Virtanen V (2013) Spatial genetic structure of aquatic bryophytes in a connected lake system. Plant Biology 15:514–521. doi:10.1111/j.1438-8677.2012.00660.x
Longton RE, Schuster RM (1983) Reproductive biology. In: Schuster RM (ed) New manual of bryology, vol 1. Hattori Botanical Laboratory, Nichinan, Japan, pp 386–462
Lynch M, Milligan BG (1994) Analysis of population genetic structure with RAPD markers. Mol Ecol 3:91–99. doi:10.1111/j.1365-294X.1994.tb00109.x
Nei M (1972) Genetic distances between populations. Ame Nat 106:283–292
Nowak C (2008) Consequences of environmental pollution on genetic diversity in populations of the midge Chironomus riparius. Dissertation, Johann Wolfgang Goethe-Universität
Peakall R, Smouse PE (2012) GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 28:2537–2539. doi:10.1093/bioinformatics/bts460
Pfeiffer T, Fritz S, Stech M, Frey W (2006) Vegetative reproduction and clonal diversity in Rhytidium rugosum (Rhytidiaceae, Bryopsida) inferred by morpho-anatomical and molecular analyses. J Plant Res 119:125–135. doi:10.1007/s10265-005-0255-x
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155(2):945–959. doi:10.1111/j.1471-8286.2007.01758.x
Reynolds JB, Weir S, Cockerham CC (1983) Estimation of the co-ancestry coefficient: basis for a short-term genetic distance. Genetics 105:767–779
Richardson DHS, Nieboer E, Lavoie P, Padovan D (1979) The role of metal-ion binding in modifying the toxic effects of sulphur dioxide on the lichen Umbilicaria muhlenbergii. II. 14C fixation studies. New Phytol 82:633–643. doi:10.1111/j.1469-8137.1979.tb01658.x
Shacklette HT (1967) Copper mosses as indicators of metal concentrations. Geol Surv Bull 1198:G1–G17
Shaw AJ, Antonovics J, Anderson LE (1987) Inter-and intra-specific variation of mosses in tolerance to copper and zinc. Evolution 41:1312–1325
Shaw AJ (1988) Genetic variation for tolerance to copper and zinc within and among populations of the moss Funaria hygrometrica. New Phytol 109:211–222. doi:10.1111/j.1469-8137.1988.tb03710.x
Shaw AJ (1990) Metal tolerances and co-tolerances in the moss Funaria hygrometrica. Can J Bot 68:2275–2282. doi:10.1139/b90-290
Spagnuolo V, Muscariello L, Terracciano S, Giordano S (2007a) Molecular biodiversity in the moss Leptodon smithii (Neckeraceae) in relation to habitat disturbance and fragmentation. J Plant Res 120:595–604. doi:10.1007/s10265-007-0097-9
Spagnuolo V, Muscariello L, Cozzolino S, Castaldo Cobianchi R, Giordano S (2007b) Ubiquitous genetic diversity in ISSR markers between and within populations of the asexually producing moss Pleurochaete squarrosa. Plant Ecol 188:91–101. doi:10.1007/s11258-006-9150-3
Spagnuolo V, Terracciano S, Giordano S (2009a) Trace element content and molecular biodiversity in the epiphytic moss Leptodon smithii: two independent tracers of human disturbance. Chemosphere 74:1158–1164. doi:10.1016/j.chemosphere.2008.11.080
Spagnuolo V, Terracciano S, Giordano S (2009b) Clonal diversity and geographic structure in Pleurochaete squarrosa: different sampling scale approach. J Plant Res 122:161–170. doi:10.1007/s10265-008-0206-4
Spagnuolo V, Zampella M, Giordano S, Adamo P (2011) Cytological stress and element uptake in moss and lichen exposed in bags in urban area. Ecotox Environ Safe 74:1434–1443. doi:10.1016/j.ecoenv.2011.02.011
Spagnuolo V, De Nicola F, Terracciano S, Bargagli R, Baldantoni D, Monaci F, Alfani A, Giordano S (2014) Persistent pollutants and the patchiness of urban green areas as drivers of genetic richness in the epiphytic moss Leptodon smithii. J Environ Sci 26:2493–2499. doi:10.1016/j.jes.2014.06.036
Staton JL, Schizas NV, Chandler GT, Coull BC, Quattro JM (2001) Ecotoxicology and population genetics: The emergence of “Phylogeographic and Evolutionary Ecotoxicology”. Ecotoxicology 10:217–222. doi:10.1023/A:1016617410786
Steinnes E, Rühling Å, Lippo H, Makinen A (1997) Reference materials for large scale metal deposition surveys. Accred. Qual Assur 2:243–249. doi:10.1007/s007690050141
Tremper AH, Agneta M, Burton S, Higgs DEB (2004) Field and laboratory exposures of two moss species to low level metal pollution. J Atmos Chem 49:111–120. doi:10.1007/s10874-004-1218-7
Tuba Z, Saxena DK, Srivastava K, Singh S, Czobel S, Kalaji HM (2010) Chlorophyll a fluorescence measurements for validating the tolerant bryophytes for heavy metal (Pb) biomapping. Curr Sci India 98:1505–1508
Ungherese G, Mengoni A, Somigli S, Baroni D, Focardi S, Ugolini A (2010) Relationship between heavy metals pollution and genetic diversity in Mediterranean populations of the sandhopper Talitrus saltator (Montagu) (Crustacea, Amphipoda). Environ Poll 158:1638–1643. doi:10.1016/j.envpol.2009.12.007
Vanderpoorten A, Tignon M (2000) Amplified fragment length polymorphism between populations of Amblystegium tenax exposed to contrasting water chemistries. J Bryology 22:257–262. doi:10.1179/jbr.2000.22.4.257
Van Straalen NM, Timmermans MJTN (2002) Genetic variation in toxicant stressed populations: an evaluation of the “genetic erosion” hypothesis. Hum Ecol Risk Assess 8:983–1002. doi:10.1080/1080-700291905783
Vekemans X (2002) AFLP-SURV version 1.0. Distributed by the author. Laboratoire de Génétique et Ecologie Végétale, Université Libre de Bruxelles, Belgium. http://ebe.ulb.ac.be/ebe/AFLP-SURV.html. Accessed 16 April 2015.
Vos P, Hogers R, Bleeker M, Reijans M, van de Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) Nucleic Acids Res 23:4407–4414
Webb DM, Knapp SJ (1990) DNA extraction from a previously recalcitrant plant genus. Plant Mol Biol Rep 8(3):180–185. doi:10.1007/BF02669514
Wells JM, Brown DH (1995) Cadmium tolerance in a metal-contaminated population of the grassland moss Rhytidiadelphus squarrosus. Ann Bot 75:21–29. doi:10.1016/S0305-7364(05)80005-8
Wright S (1931) Evolution in Mendelian populations. Genetics 16:97–159
Acknowledgments
The study was partly financed by the FEDER funding; M. T. Boquete is grateful to the Spanish Ministerio de Ciencia e Innovación for a grant awarded within the Programa de Formación de Profesorado Universitario.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Boquete, M., Spagnuolo, V., Fernández, J. et al. Genetic structuring of the moss Pseudoscleropodium purum sampled at different distances from a pollution source. Ecotoxicology 25, 1812–1821 (2016). https://doi.org/10.1007/s10646-016-1727-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-016-1727-6