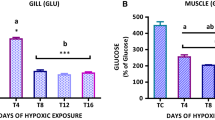
Abstract
Both hypoxia and hyperoxia, albeit in different magnitude, are known stressors in the aquatic environment. Adopting an integrated approach, mirror carp (Cyprinus carpio L.), were exposed chronically (i.e. 30 days) to hypoxic (1.8 ± 1.1 mg O2 l−1) and hyperoxic (12.3 ± 0.5 mg O2 l−1) conditions and resultant biological responses or biomarkers were compared between these two treatments as well as with fish held under normoxic conditions (7.1 ± 1.04 mg O2 l−1). The biomarkers determined included the activities of glutathione peroxidase (GPx), measurement of oxidative DNA damage (using modified Comet assay employing bacterial enzymes: Fpg and Endo-III), haematological parameters, histopathological and ultrastructural examination of liver and gills. Specific growth rate (SGR) of the fish, as an important ecotoxicological parameter was also determined over the exposure period. The study suggested that while the levels of hepatic GPx were unaffected, there was a significant difference in activity in the blood plasma under different exposure conditions; the hyperoxic group showed increased GPx activity by approximately 37% compared to normoxic group and the hypoxic group showed a decrease by approximately 38% than the normoxic group. Interestingly, oxidative DNA damage was significantly higher in both hypoxic and hyperoxic by approximately 25% compared to normoxic conditions, Fpg showing enhanced level of damage compared to the Endo-III treatment (P < 0.001). The haematological parameters showed enhanced values under hypoxic conditions. Transmission electron microscopic (TEM) studies revealed damage to liver and gill tissues for both the treatments. Interestingly, SGR of fish was significantly lowered in hypoxic by approx. 30% compared to normoxic condition and this was found to be correlated with DNA damage (R = −0.82; P = 0.02). Taken together, these results indicate that prolonged exposure to both hypoxic and hyperoxic conditions induce oxidative stress responses at both DNA and tissue levels, and hypoxia can result in compensatory changes in haematological and growth parameters which could influence Darwinian fitness of the biota with wider ecological implications.





Similar content being viewed by others
References
Au DWT, Wu RSS, S ZB, Lam PKS (1999) Relationship between ultrastructural changes and EROD activities in liver of fish exposed to Benzo[a]pyren. Environ Pollut 104:235–247
Azqueta A, Shaposhnikov S, Collins AR (2009) DNA oxidation: investigating its key role in environmental mutagenesis with the comet assay. Mutat Res 674:101–108
Belpaeme K, Cooreman K, Kirsch-Volders M (1998) Development and validation of the in vivo alkaline comet assay for detecting genomic damage in marine flatfish. Mutat Res 415:167–184
Buccellato LJ, Tso M, Akinci OI, Chandel NS, Budinger GS (2004) Reactive oxygen species are required for hyperoxia-induced bax activation and cell death in alveolar epithelial cells. J Biol Chem 279:6753–6760
Cacciuttolo MA, Trinh L, Lumpkin JA, Rao G (1993) Hyperoxia induces DNA damage in mammalian cells. Free Radic Biol Med 14:267–276
Camargo MMP, Martinez CBR (2007) Histopathology of gills, kidney and liver of a Neotropical fish caged in an urban stream. Neotrop Ichthyol 5:327–336
Cech JJ, Mitchell SJ, Wragg TE (1984) Comparative growth of juvenile white sturgeon and striped bass: effects of temperature and hypoxia. Estuaries Coasts 7:12–18
Chabot D, Dutil JD (1999) Reduced growth of Atlantic cod in non-lethal hypoxic conditions. J Fish Biol 55:472–491
Dabrowski K, Lee KJ, Guz L, Verlhac V, Gabaudan J (2004) Effects of dietary ascorbic acid on oxygen stress (hypoxia or hyperoxia), growth and tissue vitamin concentrations in juvenile rainbow trout (Oncorhynchus mykiss). Aquaculture 233:383–392
Diaz RJ, Rosenberg R (2008) Spreading dead zones and consequences for marine ecosystems. Science 32:926–929
Dirmeier R, O’Brien KM, Engle M, Dodd A, Spears E, Poyton RO (2002) Exposure of yeast cells to anoxia induces transient oxidative stress: implications for the induction of hypoxic genes. J Biol Chem 277:34773–34784
Estebani MA, Meeguer J, Garcia AA, Aglleiro B (1989) Erythropoiesis and thrombopoiesis in the head-kidney of the sea bass (Dicentrarchus labrax L.): an ultrastructural study. Arch Histol Cytol 52:407–419
Frenzilli G, Scarcelli V, Barga ID, Nigro M, Förlin L, Bolognesi C, Sturve J (2004) DNA damage in eelpout (Zoarces viviparus) from Göteborg harbour. Mutat Res 552:187–195
Glencross BD (2009) Reduced water oxygen levels affect maximal feed intake, but not protein or energy utilization efficiency of rainbow trout (Oncorhynchus mykiss). Aquac Nutr 15:1–8
Gozal E, Sachleben LR Jr, Rane MJ, Vega C, Gozal D (2005) Mild sustained and intermittent hypoxia induce apoptosis in PC-12 cells via different mechanisms. Am J Physiol 288:C535–C542
Greaney SG, Powers AD (1978) Allosteric modifiers of fish hemoglobins: in vitro and in vivo studies of the effect of ambient oxygen and pH on erythrocyte ATP concentrations. J Exp Zool 203:339–349
Guillemin K, Krasnow MA (1997) The hypoxic response: huffing and HIFing. Cell 89:9–12
Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine. Clarendon Press; Oxford University Press, Oxford New York
Handy H, Depledge MH (1999) Physiological responses: their measurement and use as environmental biomarkers in ecotoxicology. Ecotoxicology 8:329–349
Jensen FB, Weber RE (1985) Kinetics of the acclimational responses of tench to combined hypoxia and hypercapnia. J Comp Physiol B Biochem Syst Environ Physiol 156:197–203
Jha AN (2004) Genotoxicological studies in aquatic organisms: an overview. Mutat Res 552:1–17
Jha AN (2008) Ecotoxicological applications and significance of the comet assay. Mutagenesis 23:207–221
Khazanov VA, Poborskii AN (1991) Respiration of rat brain mitochondria during hyperoxia and normoxia. Bull Exp Biol Med 112:1258–1261
Kohler A (1990) Identification of contaminant-induced cellular and subcellular lesions in the liver of flounder (Platichthys flesus L.) caught at differently polluted estuaries. Aquat Toxicol 16:271–293
Kumaravel TS, Jha AN (2006) Reliable Comet assay measurements for detecting DNA damage induced by ionising radiation and chemicals. Mutat Res 605:7–16
Li CS, Wu KY, Chang-Chien GP, Chou CC (2005) Analysis of oxidative DNA damage 8-hydroxy-2′-deoxyguanosine as a biomarker of exposures to persistent pollutants for marine mammals. Environ Sci Tech 39:2455–2460
Liepelt A, Karbe L, Westendorf J (1995) Induction of DNA strand breaks in rainbow trout Oncorhynchus mykiss under hypoxic and hyperoxic conditions. Aquat Toxicol 33:177–181
Livingstone DR (2001) Contaminant-stimulated reactive oxygen species production and oxidativedamage in aquatic organisms. Mar Pollut Bull 42:656–666
Livingstone DR (2003) Oxidative stress in aquatic organisms in relation to pollution and aquaculture. Revue Méd Vét 154:427–430
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101:13–30
Lushchak VI, Bagnyukova TV (2006) Effects of different environmental oxygen levels on free radical processes in fish. Comp Biochem Physiol B Biochem Mol Biol 144:283–289
Lushchak VI, Bagnyukova TV (2007) Hypoxia induces oxidative stress in tissues of a goby, the rotan Perccottus glenii. Comp Biochem Physiol B Biochem Mol Biol 148:390–397
Lushchak VI, Lushchak LP, Mota AA, Hermes-Lima M (2001) Oxidative stress and antioxidant defenses in goldfish Carassius auratus during anoxia and reoxygenation. Am J Physiol 280:R100–R107
Lushchak VI, Bagnyukova TV, Lushchak OV, Storey JM, Storey KB (2005) Hypoxia and recovery perturb free radical processes and antioxidant potential in common carp (Cyprinus carpio) tissues. Int J Biochem Cell Biol 37:1319–1330
Lykkeboe G, Weber RE (1978) Changes in the respiratory properties of the blood in the carp, Cyprinus carpio, induced by diurnal variation in ambient oxygen tension. J Comp Physiol B 128:117–125
Mallatt J (1985) Fish gill structural changes induced by toxicants and other irritants: a statistical review. Can J Fish Aquat Sci 42:630–648
Martinovic D, Villeneuve DL, Kahl MD, Blake LS, Brodin JD, Ankley GT (2009) Hypoxia alters gene expression in the gonads of zebrafish (Danio rerio). Aquat Toxicol 95:258–272
Miller AT (2005) The role of oxygen in metabolic regulation. Helgoland Mar Res 14:392–406
Modig HG, Edgren M, Révész L (1974) Dual effect of oxygen on the induction and repair of single-strand breaks in the DNA of X-irradiated mammalian cells. Int J Radiat Biol 26:341–353
Muusze B, Marcon J, van den, Thillart G, Almeida-Val V (1998) Hypoxia tolerance of Amazon fish: respirometry and energy metabolism of the cichlid Astronotus Ocellatus. Comp Biochem Physiol A Mol Integr Physiol 120:151–156
Myers MS, Johnson LL, Hom T, Collier TK, Stein JE, Varanasi U (1998) Toxicopathic hepatic lesions in subadult English sole (pleuronectes vetuls) from Puget Sound, Washington, USA: relationships with other biomarkers of contaminant exposure. Mar Environ Res 45:47–67
Nikinmaa M (2002) Oxygen-dependent cellular functions—why fishes and their aquatic environment are a prime choice of study. Comp Biochem Physiol A Mol Integr Physiol 133:1–16
Okino ST, Chichester CH, Whitlock JP (1998) Hypoxia-inducible mammalian gene expression analyzed in vivo at aTATA-driven promoter and at an initiator-driven promoter. J Biol Chem 273:23837–23843
Poon WL, Hung CY, Nakano K, Randall DJ (2007) An in vivo study of common carp (Cyprinus carpio L.) liver during prolonged hypoxia. Comp Biochem Physiol D 2:295–302
Quintó L, Aponte JJ, Menéndez C, Sacarlal J, Aide P, Espasa M, Mandomando I, Guinovart C, Macete E, Hirt R, Urassa H, Navia MM, Thompson R, Alonso PL (2006) Relationship between haemoglobin and haematocrit in the definition of anaemia. Trop Med Int Health 11:1295–1302
Reeves JF, Davies SJ, Dodd NJF, Jha AN (2008) Hydroxyl radicals (OH) are associated with titanium dioxide (TiO2) nanoparticle-induced cytotoxicity and oxidative DNA damage in fish cells. Mutat Res 640:113–122
Rinaldia L, Patrizia B, Tettamantia G, Grimaldia A, Terovaa G, Sarogliaa, Eguileor Md (2005) Oxygen availability causes morphological changes and a different VEGF/FIk-1/HIF-2 expression pattern in sea bass gills. Ital J Zool 72:103–111
Ritola O, Livingstone DR, Peters LD, Lindström-seppä P (2002) Antioxidant processes are affected in juvenile rainbow trout (Oncorhynchus mykiss) exposed to ozone and oxygen-supersaturated water. Aquaculture 210:1–19
Scott AL, Rogers WA (1980) Histological effects of prolonged sublethal hypoxia on channel catfish Ictalurus punctatus (Rafinesque). J Fish Dis 3:305–316
Sevgi Kolayli EK (1999) A comparative study of antioxidant enzyme activities in freshwater and seawater-adapted rainbow trout. J Biochem Mol Toxicol 13:334–337
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Smit GL, Hattingh J (1978) The effect of respiratory stress on carp haemoglobin. Comp Biochem Physiol A 59:369–374
Smith RW, Houlihan DF (1995) Protein synthesis and oxygen consumption in fish cells. J Comp Physiol B 165:93–101
Soitamo AJ, Rabergh CMI, Gassmann M, Sistonen L, Nikinmaa M (2001) Characterization of a hypoxia-inducible factor (HIF-1α) from rainbow trout. Accumulation of protein occurs at normal venous oxygen tension. J Biol Chem 276:19699–19705
Soivio A, Nikinmaa M, Westman K (1980) The blood oxygen binding properties of hypoxic Salmo gairdneri. J Comp Physiol B 136:83–87
Soldatov AA (1996) The effect of hypoxia on red blood cells of flounder: a morphologic and autoradiographic study. J Fish Biol 48:321–328
Theodorakis CW, D’Surney SJ, Shugart LR (1994) Detection of genotoxic insult as DNA strand breaks in fish blood cells by agarose gel electrophoresis. Environ Toxicol Chem 13:1023–1031
Thetmeyer H, Waller U, Black KD, Inselmann S, Rosenthal H (1999) Growth of European sea bass (Dicentrarchus labrax L.) under hypoxic and oscillating oxygen conditions. Aquaculture 174:355–367
Thomas P, Rahman MS (2010) Region-wide impairment of Atlantic croaker testicular development and sperm production in the northern Gulf of Mexico hypoxic dead zone. Mar Environ Res 69:S59–S62
Valavanidis A, Vlahogianni T, Dassenakis M, Scoullos M (2006) Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol Environ Saf 64:178–189
van den Thillart G, van Waarde A (1985) Teleosts in hypoxia: aspects of anaerobic metabolism. Mol Physiol 8:393–409
van den Thillart G, Vianen G, Zaagsma J (2002) Adrenergic regulation of lipid mobilization in fishes; a possible role in hypoxia survival. Fish Physiol Biochem 27:189–204
van Raaij MTM, Breukel BJ, van den Thillart JM, Addink ADF (1994) Lipid metabolism of goldfish, (Carassius auratus L.) during normoxia and anoxia. Indications for fatty acid chain elongation. Comp Biochem Physiol B Biochem Mol Biol 107:75–84
Wedemeyer GA (1997) Effects of rearing conditions on the health and physiological quality of fish in intensive culture. In: Iwama GK, Pickering AD, Sumpter JP, Schreck CB (eds) Fish stress and health in aquaculture. Cambridge University Press, Cambridge, pp 35–71
Wu RSS (2002) Hypoxia: from molecular responses to ecosystem responses. Mar Pollut Bull 45:35–45
Acknowledgments
SAM is funded by the Ministry of Higher Education and Scientific Research, Republic of Iraq. ANJ would like to acknowledge the support received from European Regional Development Fund, INTERREG IVA (Grant No. 4059). We are thankful to Professor Andrew Collins, University of Oslo, Norway, for providing bacterial enzymes used for the modified Comet assay. Thanks are also due to Mr. Peter Russell and Mr. Benjamin Eynon for technical assistance and to Mr. Glenn Harper for help in electron microscopic studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mustafa, S.A., Al-Subiai, S.N., Davies, S.J. et al. Hypoxia-induced oxidative DNA damage links with higher level biological effects including specific growth rate in common carp, Cyprinus carpio L.. Ecotoxicology 20, 1455–1466 (2011). https://doi.org/10.1007/s10646-011-0702-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-011-0702-5