Abstract
Marine plastic litter, originating from land-based sources, enters the marine environment by passing through coastal ecosystems such as lagoons and estuaries. As early life history stages (ELHS) of many commercially important fish species rely on these transitional areas as nursery grounds, we hypothesized that they encounter a spatial gradient of habitat quality and pollution from inner to outer parts of their vital environment. With sizes < 5 mm, anthropogenic particles (AP), among them microplastic (MP) fibers and fragments, entail a high bioavailability for ELHS of fish, potentially facilitating AP uptake at early developmental stages which may have implications for their survival and growth. This study provides a contextualization baseline between feeding preferences and uptake of AP by the white seabream Diplodus sargus (Linnaeus, 1758) in an estuarine nursery ground on the southern coast of Portugal. Juvenile fish showed a generalized, omnivorous feeding mode with differences in trophic resource utilization between individuals collected at distinct seagrass meadows in the lagoon. A total of 23.13% of the fish (n = 147) were detected with AP in the gastrointestinal tract, and the mean number of AP per AP-feeding individual was 1.64 ± 1.04, with anthropogenic fibers (n = 47) occurring more frequently than fragments (n = 9). Knowledge of the underlying factors for MP ingestion will be greatly enhanced by considering environmental conditions along with species-stage and life-stage specific feeding modes and prey preferences which shape the uptake probability of anthropogenic fibers and fragments.
Similar content being viewed by others
Introduction
Coastal lagoons and estuaries are recognized as highly productive transitional environments, providing vital habitats and essential ecosystem services (Costanza et al. 1997; Beck et al. 2001; Erzini et al. 2022). Their importance as nursery grounds for many commercially valuable fish species arises from the combination of structural complexity and favorable environmental conditions (Elliott and Hemingway 2002; Seitz et al. 2014). Irrespective of their acknowledged relevance, coastal ecosystems face severe exposure to habitat degradation (Kennish 2002; Lotze et al. 2006) and pollution as a result of the continuously increasing urbanization of onshore and offshore regions (Browne et al. 2011) along with the riverine input of anthropogenic particles (AP) from land-based sources (Rochman 2018).
Within the past decade, AP, such as microplastic (MP) fragments and fibers of < 5 mm in size, became the center of scientific and public interest. Arising from the cumulative industrial application of plastic materials and the lack of efficient waste-management (Jambeck et al. 2015; Ryan 2015), increasing quantities of MP have been documented in coastal areas around the world (Barnes et al. 2009; Cole et al. 2011; Kumar et al. 2021). Due to their size range, MP particles are available for ingestion (and potential trophic transfer) for a variety of organisms at the base of the marine food web, among them early life history stages (ELHS) of fish (Cole et al. 2013; Gove et al. 2019). Growth, condition, and survival of ELHS are strongly shaped by gradients in abiotic and biotic conditions in vital nursery grounds, with direct consequences for recruitment success to adult fish stocks (Boehlert and Mundy 1988; Beverton and Iles 1992; Ciotti et al. 2014). Therefore, in-depth research on the potential uptake and consequent physiological impact of MP is needed to holistically assess the underlying factors contributing to recruitment variability in commercially important fish species. Although plastic ingestion in fish has been reported across more than 140 families (Azevedo-Santos et al. 2019), most field studies neither assessed ELHS of commercially important fish taxa nor investigated prey selectivity by comparing diet composition with prey availability (Gamito et al. 2003; Selleslagh and Amara 2015; Müller 2021). Feeding strategy and prey preferences undergo ontogenetic changes (Galarowicz et al. 2006; Sánchez-Hernández et al. 2019) and understanding of the feeding ecology of a life stage or species is considered of major importance to establish and improve sustainable management and conservation (Braga et al. 2012).
Our study aims to fill existing knowledge gaps about the potential uptake and effects of AP for growth and survival of ELHS of a commercially important fish species on the basis of a holistic examination and contextualization of the gastrointestinal tract of the fish. Juvenile white seabream, Diplodus sargus (Linnaeus, 1758), were chosen as a model organism for this field study. Due to their opportunistic prey intake and potentially disadvantageous body:AP size ratio, ELHS of fish supposedly show a higher AP ingestion probability and sensitivity towards adverse physiological effects than adults (Critchell and Hoogenboom 2018; Salerno et al. 2021). The omnivorous feeding mode of the white seabream has been hypothesized to be an influencing factor for elevated AP uptake rates (Mizraji et al. 2017; Garcia et al. 2020), yet this species is able to discriminate between natural and artificial prey items (Müller et al. 2020), challenging the abovementioned notions. The selected study area, the Ria Formosa lagoon, located at the southern Portuguese coast, is recognized as an essential nursery for the adjacent coastal fish populations, fostering significant populations of juvenile fish, including commercially relevant members of the seabream family (Sparidae) (Monteiro et al. 1990; Erzini et al. 2002; Ribeiro et al. 2006). Despite its ecological and economic importance, the ecosystem is facing persistent anthropogenic pressures (e.g., habitat degradation, impaired water quality) across a spatio-temporal gradient, with higher detrimental effects being exerted on biological communities in the interior, urbanized parts of the lagoon during summer months (Newton et al. 2003; Cravo et al. 2012; Guimarães et al. 2012).
Based on the hypotheses that the gross of AP enters the marine environment through coastal ecosystems and vegetated habitats, which are vital nursery grounds for a wide range of fish ELHS, including the white seabream, act as a trap for AP (Cozzolino et al. 2020; Jones et al. 2020), our research questions were:
-
i)
Do juvenile D. sargus encounter a gradient of habitat quality and AP pollution in their vital coastal lagoon nursery area?
-
ii)
What are the driving factors for and the potentially detrimental effects of AP ingestion for ELHS of an omnivorous fish species?
Materials and methods
Field sampling design
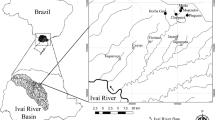
To investigate potential spatial abiotic and biotic gradients as well as differences in AP abundance, five distinct seagrass-vegetated sampling sites were selected in the western part of the Ria Formosa lagoon at the southern Portuguese coast (Fig. 1; Table 1): Two sampling sites were chosen for their proximity (linear distance < 1 km) to urbanized areas, i.e., the city center of Faro and the Praia de Faro (thereinafter called “interior 1” and “interior 2”), and two additional sampling sites were selected in intermediate linear distance (< 5 km) to the urbanized areas (“media 1” and “media 2”). One more sampling site was designated close to the inlet of the main channel to the Atlantic Ocean (“exterior”); as the only other western Ria Formosa inlet yielded no seagrass-vegetated sites to be sampled for comparison, only one station at the greatest distance to the city (linear distance ~ 7 km) was considered appropriate. The field work design included three sampling campaigns in summer 2018 (04./05. July; 27. July; 10./13. August). All samplings occurred during slack tide (max. 2 h prior and after low tide); at each site, temperature, salinity, and dissolved oxygen were recorded using a YSI Professional Plus Multiparameter Instrument (YSI Pro2030), and sampling of juvenile seabream as well as of environmental AP abundance in planktic and benthic compartments was conducted.
Map of mainland Portugal, highlighting the location of the Ria Formosa lagoon at the Algarve coast. Enlarged map of the western part of the lagoon displays the five distinct sampling sites selected for this study and two inlets. Sampling across the different stations took place between July and August 2018
Field sample collection
Seabream
Juvenile seabream were collected during all three sampling campaigns with a beach seine (25 m width, 3.5 m height, 9 mm stretched mesh size) with a cod end-type sac in the middle to facilitate handling of the catch (Erzini et al. 2002), deployed in a standardized manner (Adão et al. 2022). The net was deployed from a 6.5-m boat and towed 20 m along the shore by the boat from one side and by two to three people on the shore from the other side. After half of the towing distance, the boat headed to the shore at an angle, with the people on shore continuing towing along the shore to meet the boat when it reached the shore. Based on GPS measurements of many hauls, the average area sampled in this manner is 1087 m2 (Adão et al. 2022). The catch, which was retained in the cod end, was then emptied into a box, and sorted for seabream. Individuals that were gilled in the mesh of the seine net were also collected. Juvenile seabream were stored in labeled plastic bags inside a cooler, equipped with freezer packs, and transported to the laboratory where the fish were preserved in a freezer at CCMAR, Faro, Portugal, until further processing and analyses.
Environmental AP abundance
Zooplankton and macrozoobenthos samples were collected during the first and third sampling campaigns to assess the AP abundance in planktic and benthic compartments. AP abundance was assessed in the water column using a conical zooplankton net (200 µm mesh size, 0.13 m2 mouth opening, equipped with a HydroBios flowmeter to precisely assess the sampled water volume) which was towed 300 m just below the sea surface behind the boat around the sampling site. Additionally, macrozoobenthos samples were taken with a push net (1000 µm mesh size, rectangular opening of 20 cm height and 50 cm width), operated manually, and pushed over 10 m through the seagrass meadow. The zooplankton samples were preserved in glass jars containing a seawater-ethanol solution (70% ethanol) until further analysis. The macrozoobenthos samples were stored deep-frozen in individual zip-lock bags until further analysis.
Laboratory processing
Contamination control
Potential contamination of the samples was reduced by applying the following procedural measures (Lusher et al. 2017) in the laboratories of CCMAR in Faro, Portugal, and ZMT in Bremen, Germany: Work benches and equipment (i.e., glassware, tweezers, needles, and Bogorov chambers) were cleaned with ethanol before use, the latter being additionally checked under the microscope for contamination. Contamination with fibers was minimized by washing hands and forearms thoroughly as well as by wearing cotton laboratory coats and nitrile gloves during all analytical steps performed. Care was taken also by wearing cotton clothing underneath the laboratory coat and the color of the clothing was recorded to trace back potential contamination. If in doubt about the origin, fibers found in the samples were excluded from the analysis.
To avoid misidentification of AP, the criteria proposed by Hidalgo-Ruz et al. (2012) and Lusher et al. (2017 & 2020) were adopted to the visual sorting of fragments and fibers in the fish gastrointestinal tract (GIT) and zooplankton as well as macrozoobenthos samples—if in doubt about the origin of a fragmented or fibrous object, it was excluded from the analysis, thereby applying a conservative visual classification and quantification protocol. As no subsequent polymer characterization was performed to verify the synthetic origin of the items, the detected fragments and fibers will be referred to as anthropogenic particles.
Environmental AP abundance
Zooplankton
Zooplankton samples were transported to the laboratory facilities of ZMT, Bremen, for further analyses. In the laboratory, samples were split into 1/4–1/128 fractions (depending on the amount of zooplanktic organisms collected) using a modified Motoda plankton sub-sampler. Zooplankton and AP images with a resolution of 2400 dpi were taken from the respective subsamples with a ZooScan (ZooScan Model V4, Hydroptic Inc., France), following the procedures described by Gorsky et al. (2010). The scans obtained were analyzed using the software ZooProcess on the ImageJ macro language (Gorsky et al. 2010), allowing automated processing and measuring of the scanned images. ZooProcess associates the images with the available metadata and divides the scanned images into multiple single images ideally depicting a single organism or particle only. Images containing multiple or overlapping organisms and AP were manually edited using the software and subsequently processed again. For identification, all images were uploaded to the website EcoTaxa (http://ecotaxa.obs-vlfr.fr/prj/) where Random Forest Algorithm in combination with convolutional neural network feature extraction automatically classifies them; afterwards, the preliminary classification of potential AP, planktic organisms, and detritus was manually validated. If in doubt about the artificial origin of a fiber or fragment, the item was visually inspected both in the ZooScan scanning chamber as well as under a stereomicroscope (Gilfillan et al. 2009; Pedrotti et al. 2014). If doubt remained, the item was excluded from the analysis.
Macrozoobenthos
The entire zip-lock bag containing the benthic sampling material was placed into a sorting tray and defrosted using tap water. Upon defrosting, one or several trays were filled with either the entire sample or several subsamples (depending on the amount of material collected) to facilitate handling. In an initial step, seagrass shoots, algae, and larger prey items (e.g., broken mollusk shells, larger crustaceans) were separated from the rest of the sample and excluded from the analysis. All remaining items, AP as well as prey organisms, were manually sorted, identified, and counted.
Seabream
Fish growth
Fish were thawed at room temperature before examination. Individuals of the species Diplodus sargus were identified by visually inspecting distinctive external characteristics (i.e., pigmentation and dentition), measured to the nearest mm (standard length = SL, total length = TL, height = H) and weighed before and after dissection (WW).
Analysis of gastrointestinal tract (GIT)
The wet weight of the entire GIT was measured to the nearest mg; afterwards, the GIT was preserved in 70% ethanol and stored in Eppendorf tubes until further analysis. Upon content analysis, the GIT was put in a Bogorov counting chamber (filled with 70% ethanol) and opened with fine scissors and tweezers under a stereomicroscope (Zeiss Stemi 2000-C). The content was visually inspected for both artificial (i.e., AP fibers and fragments) and natural prey items, and the latter was identified to higher taxonomic levels following the approach of taxonomic sufficiency as proposed by Ellis (1985) and Ferraro and Cole (1990). Fibers detected in the GIT were carefully inspected for vegetal morphological features such as organic structures or segmentation to avoid misidentification with artificial fibers. Furthermore, only fibers were counted that were found attached to GIT content remains; free-floating fibers in the Bogorov counting chamber as well as fibers matching the clothing underneath the cotton lab-coat were also excluded. Though this conservative procedure could result in partial underestimation of AP ingestion, the potential bias caused by airborne contamination is reduced to a minimum to ensure reliable results. The GIT analysis time was not standardized for this study due to the variability of GIT content and volume; to enhance comparability of results, a fixed 20 × magnification was chosen and maintained throughout all analyses.
Percentage frequency of occurrence (%FO) was chosen to be an appropriate, robust measure to analyze the GIT content of fish (Baker et al. 2014); it was calculated according to the following formula:
Moreover, prey-specific abundance (%P) was calculated for all countable faunal prey items:
The analyses of the feeding strategy, prey importance, and inter-individual as well as intra-individual components of niche width were based on the two-dimensional plot (Fig. S1, supplementary information) of prey-specific abundance %P (y-axis) and frequency of occurrence %FO (x-axis), following the approach suggested by Amundsen et al. (1996) as a modification of the Costello Method (Costello 1990).
Statistical analyses
Fish morphometrics were tested for normality of distribution (Shapiro–Wilk test) and homogeneity of variance (Fligner-Killeen test for not-normally distributed data). In case of violation of normality, the Kruskal–Wallis test was used to investigate statistical differences between the groups—if significant differences were detected, Dunn’s test (with Holm correction) was computed to perform a pairwise comparison between the groups to identify which groups differ.
Prey preferences across the different stations were examined with non-metric multidimensional scaling (nMDS) ordinations, using Bray–Curtis similarity coefficient on presence-absence data (R-package: “vegan” by Oksanen et al. 2020). Analysis of similarity (ANOSIM) was computed to examine the significance of prey item groups in the ordination pattern (Clarke 1993). A subsequent indicator species analysis (R-package: “indicspecies” by De Caceres and Legendre 2009) was performed to identify prey items that were found more frequently in the GIT of fish from one station compared to another. Potential differences in the uptake of AP across sampling sites and campaigns were investigated using pairwise t-tests and Benjamini and Hochberg adjustment.
To contextualize AP uptake with other prey items, the data obtained for the MP-feeding individuals were analyzed for potential correlation by computing the Spearman rank correlation coefficient (rs) where values can vary between − 1 (indicating a strong negative correlation of the variables compared), 0 (indicating no association between the variables compared), and + 1 (indicating a strong positive correlation of the variables compared).
Redundancy analysis (RDA) was used to model the response variables (AP fragments and fibers) as a function of nominal (sampling campaign, location) and quantitative explanatory variables using PAST (Hammer et al. 2001). Three dummy variables (0, 1) were created for each of the two nominal variables (three location types and three sampling periods). The selection of quantitative variables was based on variance inflation factors (VIF), with VIF > 50 used as the criteria to remove variables. The variables retained for the RDA were low tide (m), temperature (°C), salinity (PSU), oxygen (mgl−1), wind speed (km/h), standard length (mm), wet weight (g), wet weight of the gastric intestinal tract (g), and the total number of prey items. Level of significance was set to P ≤ 0.05.
Statistical analyses and data visualization (except for RDA) were realized with Microsoft 365 and R (version 4.0.5) (R Core Team 2020).
Results
Habitat quality and environmental AP availability
All five sampling sites were vegetated by seagrass and marine algae. However, slight differences were detected in the physico-chemical characteristics of the stations (Table S1, supplementary information): Temperature ranged between 19.8 and 24.2 °C across the different sampling sites and campaigns, showing a spatio-temporal gradient from inside the lagoon to the Atlantic Ocean inlet over the different campaigns, with a minor peak during the second campaign (end of July 2018). Salinity ranged between 35.66 and 38.11 PSU, showing comparable spatio-temporal fluctuations to those for temperature. Oxygen was generally higher at all stations during the first campaign as compared to the following two samplings and ranged between 4.08 and 8.56 mgl−1, with the highest value recorded at the station closest to the Atlantic Ocean, and the lowest oxygen concentration measured at station “interior 1”.
Following a conservative visual identification using the images produced by the ZooScan and additional visual inspection, the planktic AP concentrations varied between 0.0 and 18.54 particles m−3; the lowest concentration was measured at station “exterior” during the first campaign (beginning July 2018), whereas the highest concentrations were associated with stations “interior 2” (18.54 particles m−3) and “media 2” (12.97 particles m−3) during the third sampling (mid-August 2018). The most identified AP types in plankton samples were fibers and threads (Fig. S2 A–D, supplementary information). In the macrozoobenthos samples, only a few hard AP fragments were found, which were in a size dimension larger than any prey item ingestible (> 5 cm) by the target life stage and species of this study; thus, these infrequent items were omitted from the analysis.
Due to the mismatch between planktic and benthic prey availability and the GIT contents of the fish, computation of selectivity indices such as Chesson’s α (Chesson 1978) could not be performed; consequently, the results of the zooplankton and macrozoobenthos community analyses are not detailed here. To put the results of the fish feeding preferences into perspective, however, it has to be noted that the environmental prey composition and availability (individuals m−3) showed no significant differences (ANOSIM R = − 0.14, P = 0.6995), neither between the sampling sites nor the sampling campaigns in the beginning of July and middle of August 2018.
Fish morphometrics
A total of 306 juvenile D. sargus were collected for this study. A detailed overview of the abundances and key morphometric parameters of the juvenile fish collected at the different sites at three sampling campaigns is given in Fig. 2 (see also Table S2, supplementary information). One juvenile D. sargus was collected at the station closest to the inlet to the Atlantic Ocean (“exterior”); during the first sampling campaign, this individual was smaller (SL = 15 mm) and of lighter weight (WW = 0.05 g) than all other juveniles collected at the remaining four stations. Across the three campaigns, a total of 81 white seabream were sampled at the “interior” stations (“interior 1” n = 46; “interior 2” n = 35) while the highest numbers of individuals were recorded at the intermediate stations: “Media 1” featured 65 individuals, whereas at “media 2,” 159 individuals were collected across all three sampling campaigns.
A and B Comparative overview of standard length (SL in mm) and total wet weight (in g) recorded for 306 juvenile D. sargus at five distinct sampling sites in the Ria Formosa lagoon over three sampling campaigns (1–3) in July and August 2018. Station codes: E = exterior; I = interior 1; II = interior 2; M = media 1; MM = media 2. The total number of individuals assessed at each site during each sampling campaign is indicated in brackets below the station code
During the first sampling campaign, fish SL varied between 15 and 47 mm, while the minimum recorded WW was 0.05 g, and the highest WW was 3.24 g. There was no statistically significant difference in standard length or total wet weight during the first sampling campaign among the five different stations (Dunn’s test, P > 0.05). During the second campaign, juvenile seabream were larger and heavier than during the second campaign, with SL ranging between 22 and 61 mm, and WW varying between 0.25 and 7.17 g. During the second campaign, fish collected at both “interior” stations were significantly larger and heavier in comparison to both “media” stations (Dunn’s test, P = 0.001–0.03). During the third campaign, no significant differences in standard length or total wet weight were detected among the different stations. The variation in SL was 29–62 mm, and WW ranged between 0.67 and 8.38 g.
Dietary preferences of juvenile white seabream
Prey items
The GIT analysis of a subset of white seabream (n = 147) revealed a variety of different natural prey items, dominated by crustaceans, detritus, and marine flora (algae and seagrass), along with artificial items, namely AP fibers and fragments (Table 2). Crustaceans were the most abundant prey item in frequency of occurrence (94.56%FO) and prey-specific abundance (90.45%P). Within this prey taxon, noticeable differences between %FO and %P were recorded for copepods (70.75%FO; 50.87%N), Gnathiidae (49.66%FO; 12.76%P), and decapod zoea stages (14.97%FO; 85.7%P). Comparable differences in the overall presence of a prey item in the diet and its relative abundance were also detected in other prey taxa, such as polychaetes, insects, and ascidian tadpoles (Table 2). While marine flora was present in more than 2/3 of the fish GIT, its %N was not computed as the amount of algae and plant material was numerically not quantifiable.
Feeding strategy and prey preferences
The feeding strategy and the importance of individual prey items in the diet of D. sargus are visualized in a modified Costello plot (Fig. 3) after Amundsen et al. (1996). Most prey items ingested by D. sargus are represented in the lower part of the graph. The location of the zoea stages in the upper left of the plot implies a high specific abundance and low occurrence, whereas copepods were ingested with intermediate specific abundance and high occurrence. Most prey items (except for Copepoda) were ingested with minor frequencies and abundances (prey item points located in lower left corner of the plot, including AP fibers and fragments) thus being of relatively low prey importance (dashed line). Though detritus and algae are not included in this representation due to their uncountable amount, they appeared with a high frequency of occurrence (52.38–85.03%FO) as well as in high quantities, constituting an important share of the GIT fullness.
Modified Costello plot, after Amundsen et al. (1996). Feeding strategy (solid line), niche width contribution (dotted line), and prey importance (dashed lined) of juvenile white seabream, D. sargus, are visualized based on the two-dimensional plot of prey-specific abundance (%P) and frequency of occurrence (%FO). The distribution of points along the axes and diagonals of the diagram provides the following information: specialized or generalized feeding strategy (vertical axis), rare or dominant prey items (diagonal from lower left to upper right corner), and high between-phenotype or within-phenotype contribution to the niche width (diagonal from upper left to lower right corner). Sampling of juvenile fish took place in the Ria Formosa lagoon between July and August 2018. Red asterisk = items of anthropogenic origin; yellow rhombus = Crustacea; white rhombus = polychaeta; green circle = Insecta; gray circle = Actinopterygii; gray triangle = Ascidiacea; black square = Mollusca
Although prey item consumption (Fig. 4) shows major overlaps across the different stations in the nMDS plot (stress = 0.179), weak yet significant differences in prey uptake were detected in relation to sampling site and campaign (ANOSIM R = 0.3806, P = 0.0001). According to the indicator species analysis, 8 out of 46 prey items were significantly associated with one or several groupings (P < 0.05, see supplementary information for detailed results). During the first and second campaign, fish showed a preference for Gnathiidae (P = 0.0478) across both “interior” stations and “media 1”. For juvenile white seabream collected during the third campaign at the aforementioned stations, crustacean prey items were of significant importance (P = 0.0037). Except for the first sampling in “media 1” and the one individual collected at “exterior,” algae were a key item across all sampling stations and campaigns in the diet of juvenile seabream (P < 0.0016). At “media 2,” D. sargus collected during the third campaign had a significant uptake tendency for Decapoda zoea stages (P = 0.0001), and insect prey, particularly of the hemipteran order (P = 0.0001–0.0279) which were of no importance at any other site or campaign, explaining the more distinct aggregation of this group in the nMDS plot (“media 2.3,” blue rhombus, Fig. 4).
Non-metric multidimensional scaling (nMDS) analysis ordination biplot based on Bray–Curtis coefficient of similarities between presence/absence data of prey items found in the GIT of juvenile white seabream D. sargus at five different sampling sites in the Ria Formosa lagoon (stress = 0.179, dimensions = 3, non-metric fit R2 = 0.968, linear fit R.2 = 0.799; ellipses drawn based on 95% confidence interval where applicable). Station names, e.g., “interior 1,” are extended by the sampling campaign (i.e., 1, 2, 3)
Uptake of anthropogenic particles
A total of 34 fish were found with AP in their GIT (23.13%FO). The proportion of AP-feeding individuals ranged between 13.64 and 31.03% across the four different stations located in the interior and middle sections of the Ria Formosa lagoon (Table 3), and the only white seabream collected at the station “exterior” did not have AP in its GIT. In total, 56 APs (4.19%P) were found in the GIT of the fish across the four different stations, most of them being fibers (47/56), and the remaining APs were fragments. The mean number of AP per AP-feeding fish was 1.64 ± 1.04. Most AP-feeding individuals had only one AP in their GIT (22 of 34 fish), two and three AP were found in five fish respectively, and four and five AP were detected in only one fish each. A pairwise comparison across sampling sites and campaigns revealed no statistically significant differences in the uptake of AP (pairwise t-test, adjustment: Benjamini and Hochberg, P = 0.46–0.93).
Of the AP detected in the GIT, most were of blue color (19/56), followed by black (11/56) and red (11/56). A total of eight green/blue green APs were found, additionally, four transparent fibers, two yellow fragments, and one purple fiber. Fibers were frequently found entangled within algae, detritus, and digested material (Fig. 5). However, the few, small fragments detected were also incorporated into the GIT content.
A–D Exemplary photographs showing different anthropogenic fibers and fragments detected in the GIT of juvenile white seabream, Diplodus sargus. A Green fiber entangled within algae and detritus. B Two blue fragments with partially digested zoea for size comparison. C Red fiber in digested material. D1–D3 Transparent fiber entangled within algae—separated from algae—separated and outlined in green for enhanced visibility. Scale bar = 500 µm
Contextualization of anthropogenic particle uptake
The RDA biplot is given in Fig. 6. Locations are close to the intersection of the two axes, indicating that there is no difference in fragment or fiber ingestion between locations. The number of fibers is correlated with the first axis and fish morphometrics, namely wet weight (WW), GIT wet weight (WW_GIT), and standard length (SL). The number of fragments is strongly associated with the second axis but is not strongly correlated with any of the explanatory variables. Contextualizing the presence of fibers and fragments in the GIT with other prey items (Fig. 7) revealed a strong, negative correlation between the two AP categories present in the GIT (rs = − 0.72; P ≤ 0.001). The presence of fibers in the GIT was weakly to moderately positively correlated with algae (alga 1: rs = 0.44; P ≤ 0.01; alga 2: rs = 0.34; P ≤ 0.05). Furthermore, the uptake of fibers was weakly positively correlated with the uptake of detritus (rs = 0.36; P ≤ 0.05). Due to their volume, both algae and detritus majorly contributed to the overall fullness of the GIT. Contrasting this, the presence of fragments was moderately negatively correlated to the uptake of detritus (rs = − 0.61; P ≤ 0.001) and alga 1 (rs = − 0.4; P ≤ 0.05), as well as weakly negatively correlated to the uptake of crustaceans (rs = − 0.35; P ≤ 0.05). Additionally, a weak positive correlation was detected between the presence of fragments and ascidian tadpoles in the GIT (rs = 0.35; P ≤ 0.05). No other association of AP and natural prey items was of statistical significance.
Biplot of redundancy analysis (RDA) for abundance of plastic fibers and fragments found in the gastrointestinal tracts of Diplodus sargus across three sampling location categories (exterior, media, and interior) and over three sampling campaigns in the Ria Formosa lagoon. Abbreviations: SL: standard length [mm]; Wt: total wet weight [g]; WW_GIT: wet weight of the GIT [g]; Temp: temperature [°C]; Sal: salinity [PSU]; O2: oxygen [mgl−1]; low_tide_m: depth at low tide [m]; Wind: wind speed [kmh−1]; N_Prey: total number of prey items in GIT
Correlogram of AP uptake and other key prey items, computation based on Spearman rank correlation coefficient for AP-feeding individuals of D. sargus. Negative correlation (rs − 1 to 0) is indicated by shades of red, while positive correlation (rs 0 to + 1) is given in shades of blue. Significant associations (P ≤ 0.05) are highlighted by *
Discussion
Quality control and MP identification limitations
Although the development and application of different digestion protocols (e.g., chemical digestion, enzymatic digestion) to extract MP from biological samples have been advanced over recent years, visual identification and optical analyses remain a rapid and relatively cheap method to classify and quantify plastic (Lusher et al. 2017 & 2020). However, without subsequent polymer analysis, these methodologies bear the risk of observer biases and identification inaccuracies, especially in relation to smaller MP size spectra (Lenz et al. 2015; Hanvey et al. 2017; Angelini et al. 2019). In the present study, the abovementioned risks were partially overcome by applying strict quality control measures and a conservative identification and quantification approach. By omitting fibers and fragments of questionable origin, we present a trend in AP distribution in the Ria Formosa lagoon while not claiming to depict a precise quantification of MP abundances in the respective region. The overall goal of this ecological investigation was to provide a contextualization of AP uptake in a juvenile fish species from an estuarine nursery. Thus, the visual assessment of both natural and anthropogenic prey items was necessary despite lowering the reliability of AP detection to fragments and fibers > 150 µm. Although the benefits of the ZooScan lie in the quick, semi-automated analysis of zooplankton samples, its limitations in MP detection are entailed in the depiction of images in gray-scales only (Fig. S3 A-C, supplementary information). A reliable AP identification based on color or shades (other than within the black-white-range) cannot be achieved and, therefore, requires additional visual inspection (Gilfillan et al. 2009; Pedrotti et al. 2014). Digestion of scanned subsamples, subsequent second image analysis on the ZooScan for verification along with a polymer identification, using methods such as Fourier-transform infrared spectroscopy (FTIR), would be advised for future studies aiming at precise quantification of MP concentrations in coastal ecosystems (Lins-Silva et al. 2021).
Distribution of seabream along spatio-temporal gradients of habitat quality
Members of the family Sparidae frequently use structurally complex, nearshore habitats as nursery grounds with juvenile sparids showing a high site fidelity (Erzini et al. 2002; Ribeiro et al. 2006; Abecasis et al. 2009; Vinagre et al. 2010); thus, the chosen field study design was considered appropriate to reflect potential differences in feeding preferences and AP uptake probabilities. The distribution of ELHS of white seabream in the Ria Formosa lagoon suggests a preference of this species for nursery sites inside the lagoon, which were generally characterized by higher temperatures and salinities along with lower concentrations of dissolved oxygen, and muddier, finer sediment.
The recorded fluctuations of abiotic parameters from inside the Ria Formosa lagoon to the inlet to the Atlantic Ocean verified the spatio-temporal trends previously described (Newton and Mudge 2003). As oxygen availability, salinity, and temperature significantly impact the swimming and feeding behavior of fish (Fry 1971; Blaber and Blaber 1980; Pandian and Vivekanandan 1985), the AP uptake probability may also vary in relation to these environmental parameters as indicated by our results (Fig. 6).
The slightly higher values in fish standard length and wet weight recorded at both “interior” stations at the end of July 2018 may be indicative for the variability in ingress rates into the Ria Formosa, with individuals of high body condition and thus high swimming capabilities advancing to nursery sites further inside the lagoon (Baptista et al. 2019 & 2020). However, this observation needs further verification to clearly define the significance of different micro-habitats as nursery grounds inside the Ria Formosa and thus the potential installation of customized protection and conservation measures for the white seabream.
Next to the detrimental effects of climate change scenarios, anthropogenic disturbances, or pollutants entering the Ria Formosa (Bebianno 1995; Cortesão et al. 1986; Newton et al. 2003; Newton and Mudge 2005), plastic litter represents an additional stressor to this vital ecosystem and its biological communities which has been rarely accounted for in the past (Velez et al. 2020; Cozzolino et al. 2020; Oliveira et al. 2020). Regardless of the applied conservative approach in AP quantification and the potential methodological detection limits of the ZooScan, the results of this study suggest a tendency of higher AP concentrations at stations inside the lagoon, confirming a spatial trend that has been detected in the Ria Formosa lagoon by Velez et al. (2020) as well as in other estuaries worldwide (Lima et al. 2014; Hitchcock and Mitrovic 2019). Taking the niche partitioning behavior of seabream into consideration (Sánchez-Velasco and Norbis 1997; Erzini et al. 2002; Gamito et al. 2003; Ventura et al. 2015), the AP encounter probabilities across distinct seagrass meadows and thus the potentially detrimental effects for ELHS and recruitment success may vary between different seabream species. This variability may pose a currently unexplored risk to this viable ecological and economical resource for local artisanal fisheries and aquaculture (Leitão et al. 2009; Bonanno and Orlando-Bonaca 2020).
Feeding ecology of juvenile white seabream in the Ria Formosa
The GIT content analysis revealed a generalized, omnivorous feeding mode of juvenile white seabream in the Ria Formosa: Floral, faunal, and detrital prey were ingested across the different sampling sites and campaigns, with slight differences in resource utilization detected between individuals of the different stations. Algae and seagrass were important prey items across all stations in July and August 2018, which is in line with previous studies describing the feeding preferences of white seabream (Sala and Ballesteros 1997; Figueiredo et al. 2005; Merciai et al. 2018). The importance of crustacean and insect prey organisms, which are less rapidly digested than smaller, softer items, may be slightly over-estimated (Windell and Bowen 1978; Buckland et al. 2017); however, the relevance of these prey taxa in the diet of D. sargus has also been reported before (Rosecchi 1987; Sala and Ballesteros 1997; Osman and Mahmoud 2009). Noteworthy is the presence of two fish ectoparasites, namely Gnathiidae and Caligus spp., in the GIT of the juvenile fish, further establishing evidence for a facultative cleaning behavior contributing to the trophic resource utilization of D. sargus (Rosecchi 1987; Mariani 2001; Neto et al. 2019).
Contextualization of anthropogenic particle uptake
Ichthyofaunal communities have been proven to be useful biological indicators to monitor ecosystem health and environmental quality (Whitfield and Elliott 2002; Ribeiro et al. 2008; Ramos et al. 2012). Thus, assessing the feeding ecology of ELHS of the omnivorous white seabream was considered an important first step towards a holistic understanding of the potential impacts of plastic pollution in an estuarine nursery ground. The assessment of the entire GIT content allows for the evaluation of inter-individual and species-specific prey preferences along with feeding habits potentially facilitating the disproportionate uptake of AP and enables a sound estimation of the ecological threat anthropogenic pollutants present (Ory et al. 2017; Cardozo et al. 2018; Lopes et al. 2020). As management and conservation measures rely on a sound scientific basis, the GIT analysis performed herein was complemented by the assessment of environmental parameters and AP availability, a necessary data integration only infrequently accounted for in previous studies on MP uptake by fish (Gamito et al. 2003; Cardozo et al. 2018; McGregor and Strydom 2020; Müller 2021; Wootton et al. 2021). Although several in situ studies on the feeding ecology of juvenile white seabream have been published over the past decades, MP uptake by this omnivorous species of commercial importance has been rarely investigated; thus, intraspecific comparisons of MP uptake can be drawn only to a limited extent.
Spatio-temporal factors
Across the different stations considered for this study, the proportion of individuals with AP detected in their GIT ranged between 13.64 and 31.03%FO, with a mean load of 1.64 ± 1.04 AP per AP-feeding individual. The recorded proportion of AP-feeding fish in situ is slightly higher than the MP uptake verified by a laboratory feeding experiment conducted on juveniles of the same species. In the laboratory set-up, the ingestion rate of polystyrene particles (500–1000 µm) across the different treatment groups varied between 9.8 and 17.65%FO. However, higher mean MP loads per MP-feeding individual as well as pronounced inter-individual differences in MP uptake were reported (Müller et al. 2020). A multi-species assessment conducted at the Egyptian coast found an MP-feeding proportion of 100%FO (n = 40) along with high average loads of 3593 ± 3985 particles in juvenile D. sargus (Shabaka et al. 2020). Upon closer examination of the substantial variation in the reported %FO and MP loads in juvenile D. sargus, the studies showed substantial variation in several factors previously identified to impede the comparability of findings. Next to the study/sampling characteristics (i.e., number and age/size of fish examined, sampling location), the applied quality control measures varied. Moreover, there were remarkable differences between the MP detection and quantification methodologies, as well as the MP (or AP) size ranges and types considered across the investigations (Collard et al. 2019; Markic et al. 2020).
No significant correlation between AP uptake and station was detected, presumably due to the lack of pronounced differences caused by the rather short time frame of the field study along with the high turnover rate of the water volume in the Ria Formosa during each tidal cycle. Previous investigations from estuarine environments were also not able to establish a significant correlation between MP uptake and environmental parameters even if a spatio-temporal pattern of MP ingestion was verified (e.g., Ferreira et al. 2016; Vendel et al. 2017; Silva et al. 2018).
The observation of higher loads of anthropogenic fibers than anthropogenic fragments in the GIT of the fish agrees with previous investigations reporting fibrous MP to be more abundant both in the marine realm (Rochman et al. 2015) and in fish GIT (Lusher et al. 2013; Bessa et al. 2018). Given the intense commercial fishing activity in the Ria Formosa lagoon and adjacent coastal waters, the most probable source of the fibers is fishing gear. Although the threads frequently detected in the plankton samples (Fig. S3 B + C, supplementary information) were beyond the ingestible size range for juvenile white seabream, their fragmentation products, such as smaller fiber bundles or individual fibers (Fig. 5 D1–D3), were found in the GIT of the fish. Hence, the spatial trend described for bigger sized plastics may be still considered ecologically relevant when assessing the potential risk of micro-sized AP.
Prey preferences and feeding mode
The role of feeding mode in MP uptake is still debated and no common consensus has been achieved (Mizraji et al. 2017; Markic et al. 2020). The present study detected correlations between the ingestion of fibrous AP and the presence of vegetal prey as well as detritus. The correlation between uptake of AP fibers and fish morphometrics can be explained by the fact that both prey items majorly contributed to the overall GIT fullness and GIT weight. Based on the observations made while analyzing the GIT contents, anthropogenic fibers seemed to be frequently attached or incorporated in detritus and vegetal materials, which may suggest a higher potential of herbivorous, detritivorous, or omnivorous fish species to take up synthetic fibers along with their natural, soft-bodied prey (Peters et al. 2017; van der Hal et al. 2020; Wootton et al. 2021). In benthic invertebrates, the combination of MP characteristics (i.e., size, shape) and the feeding habits of a species were more decisive in relation to MP ingestion than the trophic guild, a finding which still requires further verification for ichthyofaunal taxa (Piarulli et al. 2020). The sensory perception of prey before and during intake along with their specialized food handling apparatus, notably the different types of teeth, enables members of the sparid family to utilize a broad food spectrum. All prey items are initially sucked in, those without a carapace directly reach the pharyngeal jaws while the buccal jaws hold back hard-bodied prey items for seizing, crushing, and rejecting via the mouth (Vandewalle et al. 1995). Ontogenetic dietary shifts have been described for the white seabream, with larger individuals (> 150 mm SL) showing a tendency to ingest hard-bodied prey, such as gastropods and echinoderms (Figueiredo et al. 2005). Owing to the specialized feeding mode, ontogenetic shifts in dietary preferences may not necessarily affect the amount and type of plastic intake with varying age/size of the fish. However, smaller individuals, as the ones investigated here, frequently use vegetal prey such as algae or seagrass which potentially bear the risk of accidental ingestion of incorporated fibrous AP. The present study detected only a small number of hard-bodied natural and artificial prey items in the GIT of the juvenile white seabream, which has been found to discriminate polystyrene fragments from crustacean prey (Müller et al. 2020), further supporting the abovementioned specialization in food intake.
Considering the overall small amount of ingested AP (Table 3), as well as their rather negligible size relation in comparison to natural prey items and digested materials (Fig. 5), the overall importance of AP in the diet of juvenile white seabream appears to be marginal. This is also confirmed by the fact that neither anthropogenic fibers nor fragments were significant contributors to any observed trends in the analyses of feeding strategy and preferred prey uptake (Figs. 3 and 4).
Effects of AP ingestion for juvenile white seabream
Most studies on AP uptake in fish examined adult individuals; thus, ambiguities remain regarding the potential effects plastic pollutants exert on vulnerable early life history stages in estuarine ecosystems (Browne et al. 2011; Steer et al. 2017; Vendel et al. 2017; Critchel and Hoogenboom 2018; Müller 2021; Wootton et al. 2021). Fish morphometrics such as standard length, weight, or condition factors can be used both as an explanatory variable for AP uptake and as an indicator for potential detrimental effects (Müller 2021). In the present study, the uptake of the few AP > 150 µm was positively associated with the number of prey items ingested as well as the GIT wet weight and GIT fullness, indicating a good nutritional status of the AP-feeding fish and an accidental co-ingestion of fibers along with voluminous vegetal or detrital prey. The present study could not verify the accumulation of AP and consequent blockage of the GIT of juvenile white seabream or any detrimental effects of AP ingestion on fish condition (e.g., inferior sizes and weights of AP-feeding fish).
Especially smaller plastic items, however, and associated chemicals may be translocated to other organs and tissues, posing an ecotoxicological risk to the fish and potentially also for human health upon consumption of contaminated tissues (Rochman et al. 2014; Avio et al. 2015; Barboza et al. 2020). The arising implications, particularly for commercially relevant fish species, need further evaluation by assessing the amount of smaller-sized microplastics and nanoplastics taken up accidentally along with the food, through trophic transfer or via drinking, making use of advanced identification and quantification methodologies such as FTIR spectroscopy (Setälä et al. 2014; Roch et al. 2020; Veerasingam et al. 2020).
Conclusion
Integrative studies, considering both the feeding biology of a species and the environmental availability of plastic pollution, have the potential to enhance our understanding of the extent to which fish deliberately or unintentionally ingest AP. Despite the continuously increasing number of studies on AP ingestion by fish, the extent of AP uptake by ELHS, being crucially dependent on nursery grounds in transitional ecosystems, needs further scientific exploration. Though juvenile white seabream show an omnivorous feeding habit and may encounter a gradient of habitat quality and elevated plastic encounter rates in their nurseries, their specialized mode of prey uptake may prevent them from ingesting high concentrations of hard-bodied AP fragments. Yet, they may still be prone to taking up fibrous AP as well as smaller-sized microplastics and nanoplastics along with their natural vegetal prey or detritus. Using ichthyofaunal communities as biological indicators for AP pollution in coastal ecosystems should be realized only under consideration of species-stage and life-stage-specific feeding modes and prey preferences as these factors may affect the uptake probability of different AP shapes, sizes, and colors.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
The authors declare no competing interests.
References
Abecasis D, Bentes L, Erzini K (2009) Home range, residency and movements of Diplodus sargus and Diplodus vulgaris in a coastal lagoon: connectivity between nursery and adult habitats. Estuar Coast Shelf Sci 85:525–529. https://doi.org/10.1016/j.ecss.2009.09.001
Adão AC, Bosch NE, Bentes L et al (2022) Complementary sampling methods to improve the monitoring of coastal lagoons. Diversity 14(10):849. https://doi.org/10.3390/d14100849
Amundsen PA, Gabler HM, Staldvik F (1996) A new approach to graphical analysis of feeding strategy from stomach contents data — modification of the Costello (1990) method. J Fish Biol 48(4):607–614. https://doi.org/10.1111/j.1095-8649.1996.tb01455.x
Angelini Z, Kinner N, Thibault J et al (2019) Marine debris visual identification assessment. Mar Pollut Bull 142:69–75. https://doi.org/10.1016/j.marpolbul.2019.02.044
Avio CG, Gorbi S, Regoli F (2015) Experimental development of a new protocol for extraction and characterization of microplastics in fish tissues: first observations in commercial species from Adriatic Sea. Mar Environ Res 111:18–26. https://doi.org/10.1016/j.marenvres.2015.06.01
Azevedo-Santos VM, Gonçalves GRL, Manoel PS et al (2019) Plastic ingestion by fish: a global assessment. Environ Pollut 255(1):112994. https://doi.org/10.1016/j.envpol.2019.112994
Baker R, Buckland A, Sheaves M (2014) Fish gut content analysis: robust measure of diet composition. Fish Fish 15:170–177. https://doi.org/10.1111/faf.12026
Baptista V, Leitão F, Morais P et al (2020) Modelling the ingress of a temperate fish larva into a nursery coastal lagoon. Est Coast Shelf Sci 235:106601. https://doi.org/10.1016/j.ecss.2020.106601
Baptista V, Morais P, Cruz J et al (2019) Swimming abilities of temperate pelagic fish larvae prove that they may control their dispersion in coastal areas. Diversity 11:185. https://doi.org/10.3390/d11100185
Barboza LGA, Lopes C, Oliveira P (2020) Microplastics in wild fish from North East Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci Total Environ 717:134625. https://doi.org/10.1016/j.scitotenv.2019.134625
Barnes DKA, Galgani F, Thompson RC et al (2009) Accumulation and fragmentation of plastic debris in global environments. Philos Trans R Soc Lond, B Biol Sci 364(1526):1985–1998. https://doi.org/10.1098/rstb.2008.0205
Bebianno MJ (1995) Effects of pollutants in the Ria Formosa lagoon. Sci Total Environ 171:107–115
Beck MW, Heck KL Jr, Able KW et al (2001) The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates: a better understanding of the habitats that serve as nurseries for marine species and the factors that create site-specific variability in nursery quality will improve conservation and management of these areas. Bioscience 51(8):633–641. https://doi.org/10.1641/0006-3568(2001)051[0633:TICAMO]2.0.CO;2
Bessa F, Barría P, Neto JM et al (2018) Occurrence of microplastics in commercial fish from a natural estuarine environment. Mar Pollut Bull 128:575–584. https://doi.org/10.1016/j.marpolbul.2018.01.044
Beverton RJH, Iles TC (1992) Mortality rates of 0-group plaice (Platessa platessa L.), dab (Limanda limanda L.) and turbot (Scophthalmus maximus L.) in European waters. III. Density-dependence of mortality rates of 0-group plaice and some demographic implications. Neth J Sea Res 29:61–79. https://doi.org/10.1016/0077-7579(92)90008-3
Blaber SJM, Blaber TG (1980) Factors affecting the distribution of juvenile estuarine and inshore fish. J Fish Biol 17(2):143–162. https://doi.org/10.1111/j.1095-8649.1980.tb02749.x
Boehlert GW, Mundy BC (1988) Roles of behavioral and physical factors in larval and juvenile fish recruitment to estuarine nursery areas. Am Fish Soc Sym 3:51–67
Bonanno G, Orlando-Bonaca M (2020) Marine plastics: what risks and policies exist for seagrass ecosystems in the Plasticene? Mar Pollut Bull 158:111425. https://doi.org/10.1016/j.marpolbul.2020.111425
Braga RR, Bornatowski H, Vitule JRS (2012) Feeding ecology of fishes: an overview of worldwide publications. Rev Fish Biol Fish 22:915–929. https://doi.org/10.1007/s11160-012-9273-7
Browne MA, Crump P, Niven SJ et al (2011) Accumulation of microplastic on shorelines worldwide: sources and sinks. Environ Sci Technol 45(21):9175–9179. https://doi.org/10.1021/es201811s
Buckland A, Baker R, Loneragan N et al (2017) Standardising fish stomach content analysis: the importance of prey condition. Fish Res 196:126–140. https://doi.org/10.1016/j.fishres.2017.08.003
Cardozo ALP, Farias EGG, Rodrigues-Filho JL et al (2018) Feeding ecology and ingestion of plastic fragments by Priacanthus arenatus. What’s the fisheries contribution to the problem? Mar Pollut Bull 130:19–27. https://doi.org/10.1016/j.marpolbul.2018.03.010
Chesson J (1978) Measuring preference in selective predation. Ecology 59(2):221–215. https://doi.org/10.2307/1936364
Ciotti BJ, Targett TE, Nash RDM et al (2014) Growth dynamics of European plaice Pleuronectes platessa L. in nursery areas: a review. J Sea Res 90:64–82. https://doi.org/10.1016/j.seares.2014.02.010
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143. https://doi.org/10.1111/j.1442-9993.1993.tb00438.x
Cole M, Lindeque P, Fileman E et al (2013) Microplastic ingestion by zooplankton. Environ Sci Technol 47(12):6646–6655. https://doi.org/10.1021/es400663f
Cole M, Lindeque P, Halsband C et al (2011) Microplastics as contaminants in the marine environment: a review. Mar Poll Bull 62:2588–2597. https://doi.org/10.1016/j.marpolbul.2011.09.025
Collard F, Gasperi J, Gabrielsen GW et al (2019) Plastic particle ingestion by wild freshwater fish: a critical review. Environ Sci Technol 53:12974–12988. https://doi.org/10.1021/acs.est.9b03083
Cortesão C, Mendes R, Vale C (1986) Metais pesados em bivalves e sedimentos na Ria Formosa, Algarve. Bol Inst Nac Invest Pescas 14:3–28
Costanza R, d’Arge R, de Groot R et al (1997) The value of the world’s ecosystem services and natural capital. Nature 387:253–260. https://doi.org/10.1038/387253a0
Costello MJ (1990) Predator feeding strategy and prey importance: a new graphical analysis. J Fish Biol 36:261–263. https://doi.org/10.1111/j.1095-8649.1990.tb05601.x
Cozzolino L, Nicastro NR, Zardi GI et al (2020) Species-specific plastic accumulation in the sediment and canopy of coastal vegetated habitats. Sci Total Environ 723:138018. https://doi.org/10.1016/j.scitotenv.2020.138018
Cravo A, Pereira C, Gomes T et al (2012) A multibiomarker approach in the clam Ruditapes decussatus to assess the impact of pollution in the Ria Formosa lagoon, South Coast of Portugal. Mar Environ Res 75:23–34. https://doi.org/10.1016/j.marenvres.2011.09.012
Critchell K, Hoogenboom MO (2018) Effects of microplastic exposure on the body condition and behavior of planktivorous reef fish (Acanthochromis polyacanthus). PloS One 13(3):e0193308. https://doi.org/10.1371/journal.pone.0193308
de Caceres M, Legendre P (2009) Associations between species and groups of sites: indices and statistical inference. Ecology 90(12):3566–3574. https://doi.org/10.1890/08-1823.1
Elliott M, Hemingway KL (2002) Fishes in estuaries. Blackwell Science, London
Ellis D (1985) Taxonomic sufficiency in pollution assessment. Mar Pollut Bull 16:459
Erzini K, Bentes L, Coelho R et al (2002) Recruitment of seabreams (Sparidae) and other commercially important species in the Algarve (Southern Portugal). Final Report DG XIV/99/061, p 194
Erzini K, Parreira F, Sadat Z et al (2022) Influence of seagrass meadows on nursery and fish provisioning ecosystem services delivered by Ria Formosa, a coastal lagoon in Portugal. Ecosys Serv 58:101490. https://doi.org/10.1016/j.ecoser.2022.101490
Ferraro SP, Cole FA (1990) Taxonomic level and sample size sufficient for assessing pollution impacts on the Southern California bight macrobenthos. Mar Ecol Prog Ser 67:251–262
Ferreira GVB, Barletta M, Lima ARA et al (2016) Plastic debris contamination in the life cycle of Acoupa weakfish (Cynoscion acoupa) in a tropical estuary. ICES J Mar Sci 73(10):2695–2707. https://doi.org/10.1093/icesjms/fsw108
Figueiredo M, Morato T, Barreiros JP et al (2005) Feeding ecology of the white seabream, Diplodus sargus, and the ballan wrasse, Labrus bergylta, in the Azores. Fish Res 75:107–119. https://doi.org/10.1016/j.fishres.2005.04.013
Fry FEJ (1971) The effect of environmental factors on the physiology of fish. Fish Physiol 6:1–98
Galarowicz TL, Adams JA, Wahl DH (2006) The influence of prey availability on ontogenetic dietary shifts of a juvenile piscivore. Can J Fish Aquat Sci 63:1722–1733. https://doi.org/10.1139/F06-073
Gamito S, Pires A, Pita C et al (2003) Food availability and the feeding ecology of ichthyofauna of a Ria Formosa (South Portugal) water reservoir. Estuaries 26:938–948. https://doi.org/10.1007/BF02803352
Garcia TD, Cardozo ALP, Quirino BA et al (2020) Ingestion of microplastic by fish of different feeding habits in urbanized and non-urbanized streams in Southern Brazil. Water Air Soil Pollut 231:434. https://doi.org/10.1007/s11270-020-04802-9
Gilfillan LR, Ohman MD, Doyle MJ, Watson W (2009) Occurrence of plastic microdebris in the California Current System. Calif Coop Oceanic Fish Invest Rep 50:123–133
Gorsky G, Ohman MD, Picheral M et al (2010) Digital zooplankton image analysis using the ZooScan integrated system. J Plankton Res 32(3):285–303. https://doi.org/10.1093/plankt/fbp124
Gove JM, Whitney JL, McManus MA et al (2019) Prey-size plastics are invading larval fish nurseries. PNAS 116(48):24143–24149. https://doi.org/10.1073/pnas.1907496116
Guimarães MHME, Cunha AH, Nzinga RL et al (2012) The distribution of seagrass (Zostera noltii) in the Ria Formosa lagoon system and the implications of clam farming on its conservation. J Nat Conserv 20:30–40. https://doi.org/10.1016/j.jnc.2011.07.005
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: Paleontological Statistics Software Packagefor Education and Data Analysis. Palaeontol Electron 4(1):9
Hanvey JS, Lewis PJ, Lavers JL et al (2017) A review of analytical techniques for quantifying microplastics in sediments. Anal Methods 9:1369. https://doi.org/10.1039/c6ay02707e
Hidalgo-Ruz V, Gutow L, Thompson RC et al (2012) Microplastics in the marine environment: a review of the methods used for identification and quantification. Environ Sci Technol 46(6):3060–3075. https://doi.org/10.1021/es2031505
Hitchcock JN, Mitrovic SM (2019) Microplastic pollution in estuaries across a gradient of human impact. Environ Pollut 247:457–466. https://doi.org/10.1016/j.envpol.2019.01.069
Jambeck JR, Geyer R, Wilcox C et al (2015) Plastic waste inputs from land into the ocean. Science 347:768–771. https://doi.org/10.1126/science.1260352
Jones KL, Hartl MG, Bell MC et al (2020) Microplastic accumulation in a Zostera marina L. bed at Deerness Sound, Orkney, Scotland. Mar Pollut Bull 152:110883. https://doi.org/10.1016/j.marpolbul.2020.110883
Kennish MJ (2002) Environmental threats and environmental future of estuaries. Environ Conserv 29(1):78–107. https://doi.org/10.1017/S037689290200006
Kumar R, Sharma P, Bandyopadhyay S (2021) Evidence of microplastic in wetlands: extraction and quantification in freshwater and coastal ecosystems. J Water Process Eng 40:101966. https://doi.org/10.1016/j.jwpe.2021.101966
Leitão F, Santos MN, Erzini K et al (2009) Diplodus spp. assemblages on artificial reefs: importance for near shore fisheries. Fish Manag Ecol 16(2):88–99. https://doi.org/10.1111/j.1365-2400.2008.00646.x
Lenz R, Enders K, Stedmon CA et al (2015) A critical assessment of visual identification of marine microplastic using Raman spectroscopy for analysis improvement. Mar Pollut Bull 100(1):82–91. https://doi.org/10.1016/j.marpolbul.2015.09.026
Lima ARA, Costa MF, Barletta M (2014) Distribution patterns of microplastics within the plankton of a tropical estuary. Environ Res 132:146–155. https://doi.org/10.1016/j.envres.2014.03.031
Lins-Silva N, Marcolin CR, Kessler F et al (2021) A fresh look at microplastics and other particles in the tropical coastal ecosystems of Tamandaré, Brazil. Mar Environ Res 169:105327. https://doi.org/10.1016/j.marenvres.2021.105327
Lopes C, Raimundo J, Caetano M et al (2020) Microplastic ingestion and diet composition of planktivorous fish. Limnol Oceanogr Lett 5:103–112. https://doi.org/10.1002/lol2.10144
Lotze HK, Lenihan HS, Bourque BJ et al (2006) Depletion, degradation, and recovery potential of estuaries and coastal seas. Science 312(5781):1806–1809. https://doi.org/10.1126/science.1128035
Lusher AL, Bråte ILN, Munno K et al (2020) Is it or isn’t it: the importance of visual classification in microplastic characterization. Appl Spectrosc 74(9):1139–1153. https://doi.org/10.1177/0003702820930733
Lusher AL, McHugh M, Thompson RC (2013) Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar Pollut Bull 67:94–99. https://doi.org/10.1016/j.marpolbul.2012.11.028
Lusher AL, Welden NA, Sobral P et al (2017) Sampling, isolating and identifying microplastics ingested by fish and invertebrates. Anal Methods 9(9):1346–1360. https://doi.org/10.1039/C6AY02415G
Mariani S (2001) Cleaning behavior in Diplodus spp.: chance or choice? A hint for future investigations. J Mar Biol Assoc UK 81:715–716
Markic A, Gaertner J, Gaertner-Mazouni N et al (2020) Plastic ingestion by marine fish in the wild. Crit Rev Env Sci Tec 50:657–697. https://doi.org/10.1080/10643389.2019.1631990
McGregor S, Strydom NA (2020) Feeding ecology and microplastic ingestion in Chelon richardsonii (Mugilidae) associated with surf diatom Anaulus australis accumulations in a warm temperate South African surf zone. Mar Pollut Bull 158:111430. https://doi.org/10.1016/j.marpolbul.2020.111430
Merciai R, Rodríguez-Pietro C, Torres J et al (2018) Bioaccumulation of mercury and other trace elements in bottom-dwelling omnivorous fishes: the case of Diplodus sargus (L.) (Osteichthyes: Sparidae). Mar Pollut Bull 136:10–21. https://doi.org/10.1016/j.marpolbul.2018.08.061
Mizraji R, Ahrendt C, Perez-Venegas D et al (2017) Is the feeding type related with the content of microplastics in intertidal fish gut? Mar Pollut Bull 116(1–2):498–500. https://doi.org/10.1016/j.marpolbul.2017.01.008
Monteiro C, Lasserre G, Lam Hoai T (1990) Spatial organization of the ichthyological community in the Ria Formosa lagoon (Portugal). Oceanol Acta 13:79–96
Müller C (2021) Not as bad as it seems? A literature review on the case of microplastic uptake in fish. Front Mar Sci 8:672768. https://doi.org/10.3389/fmars.2021.672768
Müller C, Erzini K, Teodósio MA et al (2020) Assessing microplastic uptake and impact on omnivorous juvenile white seabream Diplodus sargus (Linnaeus, 1758) under laboratory conditions. Mar Pollut Bull 157:111162. https://doi.org/10.1016/j.marpolbul.2020.111162
Neto J, Vieira D, Abecasis D et al (2019) Facultative cleaning behavior of juvenile Diplodus sargus (Sparidae) and its ecological role in marine temperate waters. Mar Ecol Prog Ser 629:165–177. https://doi.org/10.3354/meps13105
Newton A, Mudge SM (2003) Temperature and salinity regimes in a shallow, mesotidal lagoon, the Ria Formosa, Portugal. Estuar Coast Shelf Sci 57:73–85. https://doi.org/10.1016/S0272-7714(02)00332-3
Newton A, Mudge SM (2005) Lagoon-sea exchanges, nutrient dynamics and water quality management of the Ria Formosa (Portugal). Estuar Coast Shelf Sci 62:405–414. https://doi.org/10.1016/j.ecss.2004.09.005
Newton A, Icely JD, Falcão M et al (2003) Evaluation of eutrophication in the Ria Formosa coastal lagoon, Portugal. Cont Shelf Res 23:1945–1961. https://doi.org/10.1016/j.csr.2003.06.008
Oksanen J, Blanchet FG, Friendly M et al (2020) vegan: community ecology package. R package version 2.5–7. https://CRAN.R-project.org/package=vegan.
Oliveira AR, Sardinha-Silva A, Andrews PLR et al (2020) Microplastics presence in cultured and wild-caught cuttlefish, Sepia officinalis. Mar Pollut Bull 160:111553. https://doi.org/10.1016/j.marpolbul.2020.111553
Ory N, Sobral P, Ferreira JL et al (2017) Amberstripe scad Decapterus muroadsi (Carangidae) fish ingest blue microplastics resembling their copepod prey along the coast of Rapa Nui (Easter Island) in the South Pacific subtropical gyre. Sci Total Environ 586:430–437. https://doi.org/10.1016/j.scitotenv.2017.01.175
Osman AM, Mahmoud HH (2009) Feeding biology of Diplodus sargus and Diplodus vulgaris (Teleostei, Sparidae) in Egyptian Mediterranean waters. World J Fish Mar Sci 1(4):290–296
Pandian TJ, Vivekanandan E (1985) Energetics of feeding and digestion. In: Tytler P, Calow P (eds) Fish energetics. Springer, Dordrecht, pp 99–124
Pedrotti ML, Bruzaud S, Dumontet B et al (2014) Plastic fragments on the surface of Mediterranean waters. In: Briand F (ed) CIESM - Marine litter in the Mediterranean and Black Seas. CIESM Workshop Monograph 46:115–123.
Peters CA, Peyton AT, Rieper KB et al (2017) Foraging preferences influence microplastic ingestion by six marine fish species from the Texas Gulf Coast. Mar Pollut Bull 124:82–88. https://doi.org/10.1016/j.marpolbul.2017.06.080
Piarulli S, Vanhove B, Comandini P et al (2020) Do different habits affect microplastics contents in organisms? A trait-based analysis on salt marsh species. Mar Pollut Bull 153:110983. https://doi.org/10.1016/j.marpolbul.2020.110983
Ramos JAA, Barletta M, Costa MF (2012) Ingestion of nylon threads by Gerreidae while using a tropical estuary as foraging grounds. Aquat Biol 17:29–34. https://doi.org/10.3354/ab00461
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Ribeiro J, Bentes L, Coelho R et al (2006) Seasonal, tidal and diurnal changes in fish assemblages in the Ria Formosa coastal lagoon (Portugal). Estuar Coast Shelf Sci 67:461–474. https://doi.org/10.1016/j.ecss.2005.11.036
Ribeiro J, Monteiro CC, Monteiro P et al (2008) Long-term changes in fish communities of the Ria Formosa coastal lagoon (southern Portugal) based on two studies made 20 years apart. Estuar Coast Shelf Sci 76:57–68. https://doi.org/10.1016/j.ecss.2007.06.001
Roch S, Friedrich C, Brinker A (2020) Uptake routes of microplastics in fishes: practical and theoretical approaches to test existing theories. Sci Rep 10:3896. https://doi.org/10.1038/s41598-020-60630-1
Rochman CM (2018) Microplastics research – from sink to source. Science 360:28–29. https://doi.org/10.1126/science.aar7734
Rochman CM, Kurobe T, Flores I et al (2014) Early warning signs of endocrine disruption in adult fish from the ingestion of polyethylene with and without sorbed chemical pollutants from the marine environment. Sci Total Environ 493:656–661. https://doi.org/10.1016/j.scitotenv.2014.06.051
Rochman CM, Tahir A, William SL et al (2015) Anthropogenic debris in seafood: plastic debris and fibers from textiles in fish and bivalves sold for human consumption. Sci Rep 5:14340. https://doi.org/10.1038/srep14340
Rosecchi E (1987) L’alimentation de Diplodus annularis, Diplodus sargus, Diplodus vulgaris et Sparus aurata (Pisces, Sparidae) dans le Golfe du Lion et les lagunes littorales. Revue Des Travaux De L’instiut De Pêches Maritimes 49:125–141
Ryan PG (2015) A brief history of marine litter research. In: Bergmann M, Gutow L, Klages M (eds) Marine anthropogenic litter. Springer, Cham, pp 1–25. https://doi.org/10.1007/978-3-319-16510-3_1
Sala E, Ballesteros E (1997) Partitioning of space and food resources by three fish of the genus Diplodus (Sparidae) in a Mediterranean rocky infralittoral ecosystem. Mar Ecol Prog Ser 152:273–283. https://doi.org/10.3354/meps152273
Salerno M, Berlino M, Mangano MC et al (2021) Microplastics and the functional traits of fishes: a global meta-analysis. Glob Change Biol 27:2645–2655. https://doi.org/10.1111/gcb.15570
Sánchez-Hernández J, Nunn AD, Adams CE et al (2019) Causes and consequences of ontogenetic dietary shifts: a global synthesis using fish models. Biol Rev 94:539–554. https://doi.org/10.1111/brv.12468
Sánchez-Velasco L, Norbis W (1997) Comparative diets and feeding habits of Boops boops and Diplodus sargus larvae, two sparid fishes co-occurring in the Northwestern Mediterranean. Bull Mar Sci 61(3):821–835
Shabaka SH, Marey RS, Ghobashy M et al (2020) Thermal analysis and enhanced visual technique for assessment of microplastics in fish from an Urban Harbor, Mediterranean Coast of Egypt. Mar Pollut Bull 159:111465. https://doi.org/10.1016/j.marpolbul.2020.111465
Seitz RD, Wennhage H, Bergström U et al (2014) Ecological value of coastal habitats for commercially and ecologically important species. ICES J Mar Sci 71(3):648–665. https://doi.org/10.1093/icesjms/fst152
Selleslagh J, Amara R (2015) Are estuarine fish opportunistic feeders? The case of a low anthropized nursery ground (the Canache Estuary, France). Estuaries Coasts 38:252–267. https://doi.org/10.1007/s12237-014-9787-4
Setälä O, Fleming-Lethinen V, Lehtiniemi M (2014) Ingestion and transfer of microplastics in the planktonic food web. Environ Pollut 185:77–83. https://doi.org/10.1016/j.envpol.2013.10.013
Silva JDB, Barletta M, Lima ARA et al (2018) Use of resources and microplastic contamination throughout the life cycle of grunts (Haemulidae) in a tropical estuary. Environ Pollut 242:1010–1021. https://doi.org/10.1016/j.envpol.2018.07.038
Steer M, Cole M, Thompson RC et al (2017) Microplastic ingestion in fish larvae in the western English Channel. Environ Pollut 226:250–259. https://doi.org/10.1016/j.envpol.2017.03.062
Vandewalle P, Saintin P, Chardon M (1995) Structures and movements of the buccal and pharyngeal jaws in relation to feeding in Diplodus sargus. J Fish Biol 46:623–656
van der Hal N, Yeruham E, Shukis D et al (2020) Uptake and incorporation of PCBs by eastern Mediterranean rabbitfish that consumed microplastics. Mar Pollut Bull 150:110697. https://doi.org/10.1016/j.marpolbul.2019.110697
Veerasingam S, Ranjani M, Venkatachalapathy R et al (2020) Contributions of Fourier transform infrared spectroscopy in microplastic pollution research: a review. Crit Rev Env Sci Tec 51(22):2681–2743. https://doi.org/10.1080/10643389.2020.1807450
Velez N, Nicastro KR, McQuaid CD et al (2020) Small scale habitat effects on anthropogenic litter material and sources in a coastal lagoon system. Mar Pollut Bull 160:111689. https://doi.org/10.1016/J.marpolbul.2020.111689
Vendel AL, Bessa F, Alves VEN et al (2017) Widespread microplastic ingestion by fish assemblages in tropical estuaries subjected to anthropogenic pressures. Mar Pollut Bull 117(1–2):448–455. https://doi.org/10.1016/j.marpolbul.2017.01.081
Ventura D, Lasinio GJ, Ardizzone G (2015) Temporal partitioning of microhabitat use among four juvenile fish species of the genus Diplodus (Pisces: Perciformes, Sparidae). Mar Ecol 36:1013–1032. https://doi.org/10.1111/maec.12198
Vinagre C, Cabral HN, Costa MJ (2010) Relative importance of estuarine nurseries for species of the genus Diplodus (Sparidae) along the Portuguese Coast. Estuar Coast Shelf Sci 86:197–202. https://doi.org/10.1016/j.ecss.2009.11.013
Whitfield AK, Elliott M (2002) Fishes as indicators of environmental and ecological changes within estuaries: a review of progress and some suggestions for the future. J Fish Bio 61:229–250. https://doi.org/10.1006/jfbi.2002.2079
Windell JT, Bowen SH (1978) Methods for study of fish diets based on analysis of stomach contents. In: Bagenal T (Ed) Methods for assessment of fish production in fresh waters. Blackwell Scientific, Oxford, pp 219–226
Wootton N, Reis-Santos P, Gillanders BM (2021) Microplastic in fish – a global synthesis. Rev Fish Biol Fisheries 31:753–771. https://doi.org/10.1007/s11160-021-09684-6
Acknowledgements
The authors sincerely thank the colleagues who helped collect data and samples in the field, in particular, Isidoro Costa and Nuno Sales Henriques. The authors would like to acknowledge the support both in the field and in the laboratory by technicians, foremost Stefanie Bröhl and Constanze von Waldthausen, as well as student assistants, especially Elisa Gunske and Max Ratusinski. We thank all colleagues for their comments on earlier versions of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was funded by Portuguese national funds from FCT—Foundation for Science and Technology through projects UIDB/04326/2020, UIDP/04326/2020 and LA/P/0101/2020 to CCMAR. Work by the corresponding author was co-sponsored by the PhD Fellowship of the Heinrich-Böll-Stiftung, Berlin and the JPI-Oceans MicroplastiX Project (grant number 03F0852B).
Author information
Authors and Affiliations
Contributions
Carolin Müller: conceptualization, methodology, validation, formal analysis, investigation, writing—original draft, visualization, funding acquisition. Karim Erzini: supervision, conceptualization, methodology, validation, investigation, resources, writing—review and editing, project administration, funding acquisition. Tim Dudeck: formal analysis, investigation, writing—review and editing, visualization. Joana Cruz: validation, investigation, writing—review and editing. Luana Santos Corona: investigation, writing—review and editing. Felipe Eloy Abrunhosa: investigation, writing—review and editing. Carlos Manuel Lourenço Afonso: validation, investigation, writing—review and editing. Miguel Ângelo Franco Mateus: validation, investigation, writing—review and editing. Cristina Orro: investigation, writing—review and editing. Pedro Monteiro: investigation, writing—review and editing. Werner Ekau: supervision, conceptualization, methodology, validation, resources, writing—review and editing, project administration, funding acquisition.
Corresponding author
Ethics declarations
Ethical approval
The collection of samples was approved by the Instituto da Conservação da Natureza e das Florestas for Carolin Müller, Karim Erzini, and Isidoro Costa. Treatment of samples was under the Animal Care regulations at the respective research institutions.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Müller, C., Erzini, K., Dudeck, T. et al. Variability of prey preferences and uptake of anthropogenic particles by juvenile white seabream in a coastal lagoon nursery ground. Environ Biol Fish 106, 1383–1404 (2023). https://doi.org/10.1007/s10641-023-01423-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-023-01423-z