Summary
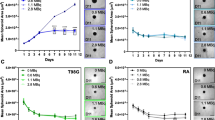
To address the major medical need for effective chemotherapeutics/diagnostics for brain cancer, in this work three cyclopentadienyl M(CO)3+ (M = Re, 99mTc) complexes, which cross the blood-brain barrier (BBB) in high % and are designed to mimic the anticancer agent 2-phenylbenzothiazole, are in vitro and in vivo evaluated for anticancer action. The study includes cytotoxicity and uptake studies in cancer and healthy neuronal cell lines, mechanistic investigation of potential anticancer pathways, and biodistribution studies in mice bearing glioblastoma xenografts. The stable Re complexes exhibit selective uptake and significant antiproliferative effect, particularly against U-251 MG glioblastoma cells, with no significant toxicity in healthy neurons, demonstrating the suitability of this type of complexes to serve as selective therapeutic/imaging agents for brain cancer. Furthermore, they result in the generation of elevated Reactive Oxygen Species (ROS) levels, and lead to significant G2/M arrest followed by apoptosis. Biodistribution studies in U-251 MG xenograft bearing mice with the radioactive 99mTc complex that exhibits the highest BBB penetration, show retention at the tumor-site offering a diagnostic prospect and, in addition, indicating the capability of the Re analogue to accumulate at the tumor site for therapeutic action. Overall, the complexes demonstrate significant anticancer properties that, combined with their high BBB penetration potential, render them strong candidates for further evaluation as brain cancer agents.



Similar content being viewed by others
Availability of data and materials
All data are included in this article.
References
Tan AC et al (2020) Management of glioblastoma: State of the art and future directions. CA Cancer J Clin 70(4):299–312. https://doi.org/10.3322/caac.21613
Ostrom QT et al (2018) CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro Oncol 20(suppl_4):iv1–iv86. https://doi.org/10.1093/neuonc/noy131
Aldape K et al (2019) Challenges to curing primary brain tumours. Nat Rev Clin Oncol 16(8):509–520. https://doi.org/10.1038/s41571-019-0177-5
Fortin D (2012) The blood-brain barrier: its influence in the treatment of brain tumors metastases. Curr Cancer Drug Targets 12(3):247–259. https://doi.org/10.2174/156800912799277511
Weidle UH, Niewohner J, Tiefenthaler G (2015) The blood–brain barrier challenge for the treatment of brain cancer, secondary brain metastases, and neurological diseases. Cancer Genomics Proteomics 12(4):167–177
Gao H, Jiang X (2013) Progress on the diagnosis and evaluation of brain tumors. Cancer Imaging 13(4):466–481. https://doi.org/10.1102/1470-7330.2013.0039
Sagnou M et al (2019) Remarkable Brain Penetration of Cyclopentadienyl M(CO)(3)(+) (M=Tc-99m, Re) Derivatives of Benzothiazole and Benzimidazole Paves the Way for Their Application as Diagnostic, with Single-Photon-Emission Computed Tomography (SPECT), and Therapeutic Agents for Alzheimer’s Disease. J Med Chem 62(5):2638–2650. https://doi.org/10.1021/acs.jmedchem.8b01949
Pelecanou M et al (2019) Tricarbonyl complexes of transition metals with benzo-heterocyclic derivatives of the cyclopentadienyl anion EP Office, Editor
Boschi A, Uccelli L, Martini P (2019) A picture of modern Tc-99m radiopharmaceuticals: Production, chemistry, and applications in molecular imaging. Appl Sci Basel 9(12). https://doi.org/10.3390/App9122526
Uccelli L et al (2019) Therapeutic radiometals: worldwide scientific literature trend analysis (2008–2018). Molecules 24(3). https://doi.org/10.3390/Molecules24030640
Jurgens S, Herrmann WA, Kuhn FE (2014) Rhenium and technetium based radiopharmaceuticals: Development and recent advances. J Organomet Chem 751:83–89. https://doi.org/10.1016/j.jorganchem.2013.07.042
Keri RS et al (2015) A comprehensive review in current developments of benzothiazole-based molecules in medicinal chemistry. Eur J Med Chem 89:207–251. https://doi.org/10.1016/j.ejmech.2014.10.059
Sharma PC et al (2013) Medicinal significance of benzothiazole scaffold: an insight view. J Enzyme Inhib Med Chem 28(2):240–266. https://doi.org/10.3109/14756366.2012.720572
Frei A, Spingler B, Alberto R (2018) Multifunctional cyclopentadienes as a scaffold for combinatorial bioorganometallics in [((5)-C5H2R1R2R3)M(CO)(3)] (M=Re, Tc-99m) piano-stool complexes. Chem Eur J 24(40):10156–10164. https://doi.org/10.1002/chem.201801271
Stone EL et al (2015) Antitumour benzothiazoles. Part 32: DNA adducts and double strand breaks correlate with activity; synthesis of 5F203 hydrogels for local delivery. Bioorg Med Chem 23(21):6891–6899. https://doi.org/10.1016/j.bmc.2015.09.052
Bradshaw TD, Westwell AD (2004) The development of the antitumour benzothiazole prodrug, Phortress, as a clinical candidate. Curr Med Chem 11(8):1009–1021. https://doi.org/10.2174/0929867043455530
Bradshaw TD, Stevens MF, Westwell AD (2001) The discovery of the potent and selective antitumour agent 2-(4-amino-3-methylphenyl)benzothiazole (DF 203) and related compounds. Curr Med Chem 8(2):203–210. https://doi.org/10.2174/0929867013373714
Halevas E et al (2020) Structurally characterized gallium-chrysin complexes with anticancer potential. Dalton Trans 49(8):2734–2746. https://doi.org/10.1039/c9dt04540f
Mavroidi B et al (2016) Palladium(II) and platinum(II) complexes of derivatives of 2-(4 ’-aminophenyl)benzothiazole as potential anticancer agents. Inorg Chim Acta 444:63–75. https://doi.org/10.1016/j.ica.2016.01.012
Tzanopoulou S et al (2010) Evaluation of Re and Tc-99m Complexes of 2-(4 ’-Aminophenyl)benzothiazole as Potential Breast Cancer Radiopharmaceuticals. J Med Chem 53(12):4633–4641. https://doi.org/10.1021/jm1001293
Islam MK et al (2021) Synthesis, characterization, and anticancer activity of benzothiazole aniline derivatives and their platinum (II) complexes as wew chemotherapy agents. Pharmaceuticals (Basel) 14(8). https://doi.org/10.3390/ph14080832
Sultana F et al (2019) Synthesis of 2-anilinopyridyl linked benzothiazole hydrazones as apoptosis inducing cytotoxic agents. New J Chem 43(18):7150–7161. https://doi.org/10.1039/c8nj06517a
Hegde M et al (2017) A Benzothiazole Derivative (5g) induces DNA damage and potent G2/M arrest in cancer cells. Sci Rep 7. https://doi.org/10.1039/S41598-017-02489
Lei Q et al (2015) A novel benzothiazole derivative SKLB826 inhibits human hepatocellular carcinoma growth via inducing G2/M phase arrest and apoptosis. RSC Adv 5(52):41341–41351. https://doi.org/10.1039/c5ra05387k
Irfan A et al (2020) Benzothiazole derivatives as anticancer agents. J Enzyme Inhib Med Chem 35(1):265–279. https://doi.org/10.1080/14756366.2019.1698036
Dragojevic, S., R. Mackey, and D. Raucher, Evaluation of elastin-like polypeptides for tumor targeted delivery of doxorubicin to glioblastoma. Molecules 24(18). https://doi.org/10.3390/molecules24183242
Taghavi MS et al (2013) Cisplatin downregulates BCL2L12, a novel apoptosis-related gene, in glioblastoma cells. In Vitro Cell Dev Biol Anim 49(6):465–472. https://doi.org/10.1007/s11626-013-9622-4
Farooqi AA et al (2015) Anticancer drugs for the modulation of endoplasmic reticulum stress and oxidative stress. Tumour Biol 36(8):5743–5752. https://doi.org/10.1007/s13277-015-3797-0
Aggarwal V et al (2019) Role of reactive oxygen species in cancer progression: Molecular mechanisms and recent advancements. Biomolecules 9(11). https://doi.org/10.3390/biom9110735
Yokoyama C et al (2017) Induction of oxidative stress by anticancer drugs in the presence and absence of cells. Oncol Lett 14(5):6066–6070. https://doi.org/10.3892/ol.2017.6931
Perillo B et al (2020) ROS in cancer therapy: the bright side of the moon. Exp Mol Med 52(2):192–203. https://doi.org/10.1038/s12276-020-0384-2
Bates D, Eastman A (2017) Microtubule destabilising agents: far more than just antimitotic anticancer drugs. Br J Clin Pharmacol 83(2):255–268. https://doi.org/10.1111/bcp.13126
DiPaola RS (2002) To arrest or not to G(2)-M Cell-cycle arrest : commentary re: AK Tyagi et al., Silibinin strongly synergizes human prostate carcinoma DU145 cells to doxorubicin-induced growth inhibition, G(2)-M arrest, and apoptosis. Clin Cancer Res 8:3512–3519 2002. Clin Cancer Res 8(11): 3311–3314
Markovits J et al (1989) Inhibitory effects of the tyrosine kinase inhibitor genistein on mammalian DNA topoisomerase II. Cancer Res 49(18):5111–5117
Ouyang L et al (2012) Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif 45(6):487–498. https://doi.org/10.1111/j.1365-2184.2012.00845.x
Hickman JA (1992) Apoptosis induced by anticancer drugs. Cancer Metastasis Rev 11(2):121–139. https://doi.org/10.1007/BF00048059
Rathinam R et al (2015) Cisplatin-induced apoptosis in auditory, renal, and neuronal cells is associated with nitration and downregulation of LMO4. Cell Death Discov 1. https://doi.org/10.1038/cddiscovery.2015.52
Pellegata NS et al (1996) DNA damage and p53-mediated cell cycle arrest: a reevaluation. Proc Natl Acad Sci U S A 93(26):15209–15214. https://doi.org/10.1073/pnas.93.26.15209
Xia W et al (2000) Tumor selective G2/M cell cycle arrest and apoptosis of epithelial and hematological malignancies by BBL22, a benzazepine. Proc Natl Acad Sci U S A 97(13):7494–7499. https://doi.org/10.1073/pnas.97.13.7494
Vermeulen K, Berneman ZN, Van Bockstaele DR (2003) Cell cycle and apoptosis. Cell Prolif 36(3):165–175. https://doi.org/10.1046/j.1365-2184.2003.00267.x
Han B-I, Lee M (2015) Paclitaxel-induced G2/M arrest via different mechanism of actions in glioma cell lines with differeing p53 mutational status. Int J Pharmacol 12:19–27
Glantz MJ et al (1995) Paclitaxel disposition in plasma and central nervous systems of humans and rats with brain tumors. J Natl Cancer Inst 87(14):1077–1081. https://doi.org/10.1093/jnci/87.14.1077
Taylor OG, Brzozowski JS, Skelding KA (2019) Glioblastoma multiforme: An overview of emerging therapeutic targets. Front Oncol 9:963. https://doi.org/10.3389/fonc.2019.00963
Liu EK et al (2020) Novel therapies for glioblastoma. Curr Neurol Neurosci Rep 20(7):19. https://doi.org/10.1007/s11910-020-01042-6
Lee SY (2016) Temozolomide resistance in glioblastoma multiforme. Genes Dis 3(3):198–210. https://doi.org/10.1016/j.gendis.2016.04.007
Shen W, Hu JA, Zheng JS (2014) Mechanism of temozolomide-induced antitumour effects on glioma cells. J Int Med Res 42(1):164–172. https://doi.org/10.1177/0300060513501753
Nosrati S et al (2020) Tc-99m-radiolabeled imidazo[2,1-b]benzothiazole derivatives as potential radiotracers for glioblastoma. J Radioanal Nucl Chem 323(1):205–211. https://doi.org/10.1007/s10967-019-06945-4
Schillaci O et al (2007) Single-photon emission computed tomography/computed tomography in brain tumors. Semin Nucl Med 37(1):34–47. https://doi.org/10.1053/j.semnuclmed.2006.08.003
Acknowledgements
B. Mavroidi gratefully acknowledges the financial support by the State Scholarships Foundation (ΙΚΥ) through the implementation of the Operational Programme «Human Resources Development, Education and Lifelong Learning» in the context of the project “Reinforcement of Postdoctoral Researchers -2nd Cycle” (MIS-5033021), co-financed by Greece and the European Union (European Social Fund-ESF).
Funding
This research is co-financed by Greece and the European Union (European Social Fund-ESF) through the Operational Programme «Human Resources Development, Education and Lifelong Learning» in the context of the project “Reinforcement of Postdoctoral Researchers 2nd Cycle” (MIS-5033021), implemented by the State Scholarships Foundation (ΙΚΥ).
Author information
Authors and Affiliations
Contributions
All authors contributed to the present study and all read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was conducted in accordance with the Presidential Decree 56/2013 (published in the Official Government Gazette of Greece 106 A/30–4-2013) that has transposed the EU Directive 2010/63 on the protection of animals used for scientific purposes.
Consent to participate
Not applicable.
Consent for publication
All authors gave consent to publication of this study.
Research involving animals
All procedures performed in studies involving animals were in accordance with the Presidential Decree 56/2013 (published in the Official Government Gazette of Greece 106 A/30–4-2013) that has transposed the EU Directive 2010/63 on the protection of animals used for scientific purposes.
Conflicts of interest
The authors do not have any potential conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mavroidi, B., Kaminari, A., Makrypidi, K. et al. Biological evaluation of complexes of cyclopentadienyl M(CO)3+ (M = Re, 99mTc) with high blood–brain barrier penetration potential as brain cancer agents. Invest New Drugs 40, 497–505 (2022). https://doi.org/10.1007/s10637-022-01211-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-022-01211-z