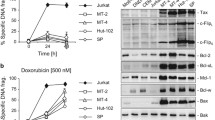
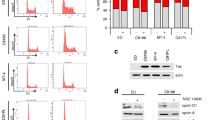
Summary
Human T cell leukemia virus type 1 (HTLV-1) is responsible for adult T cell leukemia (ATL); however, molecular and cellular mechanisms underlying HTLV-1-induced leukemogenesis are unclear. BCL6 oncogene is involved in cancer progression and a preferred target of anti-cancer treatments. Here, we aimed to evaluate BCL6 expression and the effects of BCL6 inhibitor (FX1) on HTLV-1-infected T cell lines. BCL6 expression was higher in HTLV-1-infected T cell lines than that in uninfected T cell lines. BCL6 was localized mostly in the nucleus. The virus oncoprotein Tax induced BCL6 mRNA expression in T cells, whereas BCL6 knockdown reduced HTLV-1-infected T cell proliferation; thus, confirmed the association of BCL6 with cancer progression. Further, FX1 efficiently inhibited the cell growth and survival of HTLV-1-infected T cell lines in a dose- and time-dependent manner. The decreased levels of cell cycle regulatory proteins (phosphorylated retinoblastoma protein, cyclin-dependent kinase 4, cyclin D2 and c-Myc) and the increased levels of BCL6 target proteins (p21, p27 and p53) showed that FX1 arrested cell cycle at the G1 phase. Apoptosis was induced concomitantly with Bak upregulation and downregulation of survivin, Bcl-xL and Mcl-1, as well as with the activation of caspase-3, -8, -9 and poly(ADP-ribose) polymerase. FX1 also inhibited NF-κB and Akt signaling pathways. These events were because of the induction of the activity of cell cycle checkpoint proteins and relief of direct repression of the targets of cell cycle checkpoint proteins. Thus, BCL6 might be considered a novel target for ATL treatment.






Similar content being viewed by others
Data availability
The data and materials used in this study are available from the corresponding author on reasonable request.
References
Iwanaga M, Watanabe T, Yamaguchi K (2012) Adult T-cell leukemia: a review of epidemiological evidence. Front Microbiol 3:322
Nasr R, Marçais A, Hermine O, Bazarbachi A (2017) Overview of targeted therapies for adult T-cell leukemia/lymphoma. Methods Mol Biol 1582:197–216
Ishitsuka K, Tamura K (2014) Human T-cell leukaemia virus type I and adult T-cell leukaemia-lymphoma. Lancet Oncol 15:e517–e526
Matsuoka M (2005) Human T-cell leukemia virus type I (HTLV-I) infection and the onset of adult T-cell leukemia (ATL). Retrovirology 2:27
Yamagishi M, Watanabe T (2012) Molecular hallmarks of adult T cell leukemia. Front Microbiol 3:334
Mohanty S, Harhaj EW (2020) Mechanisms of oncogenesis by HTLV-1 Tax. Pathogens 9:543
Cardenas MG, Oswald E, Yu W, Xue F, MacKerell AD Jr, Melnick AM (2017) The expanding role of the BCL6 oncoprotein as a cancer therapeutic target. Clin Cancer Res 23:885–893
Li S, Wang Z, Lin L, Wu Z, Yu Q, Gao F, Zhang J, Xu Y (2019) BCL6 rearrangement indicates poor prognosis in diffuse large B-cell lymphoma patients: a meta-analysis of cohort studies. J Cancer 10:530–538
Miyoshi I, Kubonishi I, Yoshimoto S, Akagi T, Ohtsuki Y, Shiraishi Y, Nagata K, Hinuma Y (1981) Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature 294:770–771
Yamamoto N, Okada M, Koyanagi Y, Kannagi M, Hinuma Y (1982) Transformation of human leukocytes by cocultivation with an adult T cell leukemia virus producer cell line. Science 217:737–739
Koeffler HP, Chen IS, Golde DW (1984) Characterization of a novel HTLV-infected cell line. Blood 64:482–490
Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC (1980) Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA 77:7415–7419
Miyoshi I, Kubonishi I, Sumida M, Hiraki S, Tsubota T, Kimura I, Miyamoto K, Sato J (1980) A novel T-cell line derived from adult T-cell leukemia. Gan 71:155–156
Ohtani K, Nakamura M, Saito S, Nagata K, Sugamura K, Hinuma Y (1989) Electroporation: application to human lymphoid cell lines for stable introduction of a transactivator gene of human T-cell leukemia virus type I. Nucleic Acids Res 17:1589–1604
Zhang C, Ao Z, Seth A, Schlossman SF (1996) A mitochondrial membrane protein defined by a novel monoclonal antibody is preferentially detected in apoptotic cells. J Immunol 157:3980–3987
Tanaka Y, Yoshida A, Takayama Y, Tsujimoto H, Tsujimoto A, Hayami M, Tozawa H (1990) Heterogeneity of antigen molecules recognized by anti-tax1 monoclonal antibody Lt-4 in cell lines bearing human T cell leukemia virus type I and related retroviruses. Jpn J Cancer Res 81:225–231
Mori N, Ishikawa C, Senba M (2015) Activation of PKC-δ in HTLV-1-infected T cells. Int J Oncol 46:1609–1618
Kataoka K, Nagata Y, Kitanaka A, Shiraishi Y, Shimamura T, Yasunaga J, Totoki Y, Chiba K, Sato-Otsubo A, Nagae G, Ishii R, Muto S, Kotani S, Watatani Y, Takeda J, Sanada M, Tanaka H, Suzuki H, Sato Y, Shiozawa Y, Yoshizato T, Yoshida K, Makishima H, Iwanaga M, Ma G, Nosaka K, Hishizawa M, Itonaga H, Imaizumi Y, Munakata W, Ogasawara H, Sato T, Sasai K, Muramoto K, Penova M, Kawaguchi T, Nakamura H, Hama N, Shide K, Kubuki Y, Hidaka T, Kameda T, Nakamaki T, Ishiyama K, Miyawaki S, Yoon SS, Tobinai K, Miyazaki Y, Takaori-Kondo A, Matsuda F, Takeuchi K, Nureki O, Aburatani H, Watanabe T, Shibata T, Matsuoka M, Miyano S, Shimoda K, Ogawa S (2015) Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat Genet 47:1304–1315
Arguni E, Arima M, Tsuruoka N, Sakamoto A, Hatano M, Tokuhisa T (2006) JunD/AP-1 and STAT3 are the major enhancer molecules for high Bcl6 expression in germinal center B cells. Int Immunol 18:1079–1089
Gazon H, Barbeau B, Mesnard JM, Peloponese JM Jr (2018) Hijacking of the AP-1 signaling pathway during development of ATL. Front Microbiol 8:2686
Inoue J, Seiki M, Taniguchi T, Tsuru S, Yoshida M (1986) Induction of interleukin 2 receptor gene expression by p40x encoded by human T-cell leukemia virus type 1. EMBO J 5:2883–2888
Cardenas MG, Yu W, Beguelin W, Teater MR, Geng H, Goldstein RL, Oswald E, Hatzi K, Yang SN, Cohen J, Shaknovich R, Vanommeslaeghe K, Cheng H, Liang D, Cho HJ, Abbott J, Tam W, Du W, Leonard JP, Elemento O, Cerchietti L, Cierpicki T, Xue F, MacKerell AD Jr, Melnick AM (2016) Rationally designed BCL6 inhibitors target activated B cell diffuse large B cell lymphoma. J Clin Invest 126:3351–3362
Bretones G, Delgado MD, León J (2015) Myc and cell cycle control. Biochim Biophys Acta 1849:506–516
Zheng H-C (2017) The molecular mechanisms of chemoresistance in cancers. Oncotarget 8:59950–59964
Mori N (2009) Cell signaling modifiers for molecular targeted therapy in ATLL. Front Biosci 14:1479–1489
Goldar S, Khaniani MS, Derakhshan SM, Baradaran B (2015) Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac J Cancer Prev 16:2129–2144
Hoffman B, Liebermann DA (2008) Apoptotic signaling by c-MYC. Oncogene 27:6462–6472
Pahl HL (1999) Activators and target genes of Rel/NF-κB transcription factors. Oncogene 18:6853–6866
Iwanaga R, Ohtani K, Hayashi T, Nakamura M (2001) Molecular mechanism of cell cycle progression induced by the oncogene product Tax of human T-cell leukemia virus type I. Oncogene 20:2055–2067
Reuter S, Prasad S, Phromnoi K, Ravindran J, Sung B, Yadav VR, Kannappan R, Chaturvedi MM, Aggarwal BB (2010) Thiocolchicoside exhibits anticancer effects through downregulation of NF-κB pathway and its regulated gene products linked to inflammation and cancer. Cancer Prev Res 3:1462–1472
Viatour P, Merville M-P, Bours V, Chariot A (2005) Phosphorylation of NF-κB and IκB proteins: implications in cancer and inflammation. Trends Biochem Sci 30:43–52
Shao J, Fujiwara T, Kadowaki Y, Fukazawa T, Waku T, Itoshima T, Yamatsuji T, Nishizaki M, Roth JA, Tanaka N (2000) Overexpression of the wild-type p53 gene inhibits NF-κB activity and synergizes with aspirin to induce apoptosis in human colon cancer cells. Oncogene 19:726–736
Kawauchi K, Araki K, Tobiume K, Tanaka N (2008) p53 regulates glucose metabolism through an IKK-NF-κB pathway and inhibits cell transformation. Nat Cell Biol 10:611–618
Levine AJ, Puzio-Kuter AM (2010) The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science 330:1340–1344
Acknowledgements
We acknowledge the Fujisaki Cell Center, Hayashibara Biochemical Laboratories, Inc. for providing HUT-102, MT-1 and Jurkat cells, Dr. Naoki Yamamoto (Tokyo Medical and Dental University) for providing MT-2, MT-4 and MOLT-4 cells, Dr. Masahiro Fujii (Niigata University) for providing CCRF-CEM cells, and Dr. Diane Prager (UCLA School of Medicine) for providing SLB-1 cells. Protein concentration measurement and flow cytometry analyses were performed at the University of the Ryukyus Center for Research Advancement and Collaboration. We thank Editage (www.editage.com) for their assistance with English language editing.
Funding
The study was supported in part by JSPS KAKENHI (17K07175).
Author information
Authors and Affiliations
Contributions
Conceptualization: N.M.; methodology: C.I. and N.M.; formal analysis and investigation: C.I. and N.M.; writing-original draft preparation: C.I.; writing-reviewing and editing: N.M.; funding acquisition: C.I.; resources: N.M.; supervision: N.M.
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Informed consent
Not applicable.
Consent for publication
All authors consent to the publication of this study.
Research involving human participants and/or animals
Not applicable.
Conflict of interests
No potential competing interest is reported by the authors. No potential conflict of interest is reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ishikawa, C., Mori, N. FX1, a BCL6 inhibitor, reactivates BCL6 target genes and suppresses HTLV-1-infected T cells. Invest New Drugs 40, 245–254 (2022). https://doi.org/10.1007/s10637-021-01196-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-021-01196-1