Abstract
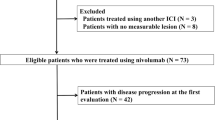
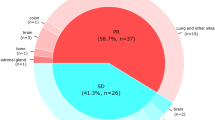
Immune checkpoint inhibitors (ICIs) are effective for previously treated patients with advanced non-small cell lung cancer (NSCLC). However, an unconventional response pattern is sometimes encountered. A dissociated response (DR), characterized by some lesions shrinking and others growing, has been recognized with ICI treatment. In this study, we examined the characteristics and treatment outcomes of DR in previously treated NSCLC patients, receiving nivolumab monotherapy. We conducted a retrospective cohort study of previously treated patients with advanced NSCLC who received nivolumab. We assessed the tumor response of each organ using the Response Evaluation Criteria in Solid Tumors (RECIST) criteria at the first radiologic evaluation. We investigated treatment outcome and compared overall survival using the Kaplan-Meier Method and log-rank tests. Further, we conducted the same analysis in patients who had previously received chemotherapy or tyrosine kinase inhibitor therapy in our hospital. Between April 2016 and September 2018, 107 patients who received nivolumab fulfilled the inclusion criteria. Of them, 5 (5%) patients showed a DR. There were no specific differences in characteristics between DR and non-DR cases. Patients showing DR had significantly longer overall survival than those showing concordant progressive disease (46.9 vs. 8.2 months, p = 0.038). The frequencies of DR in the ICI, chemotherapy, and tyrosine kinase inhibitor-treated cohorts were 5%, 1%, and 4%, respectively. DR was uncommon, but this presented a distinctive pattern of nivolumab response. Some patients might benefit from continuing nivolumab therapy and may achieve a longer overall survival.






Similar content being viewed by others
Data availability
All datasets on which the conclusions of this paper rely are available on request.
Abbreviations
- CR:
-
Complete response
- DR:
-
Dissociated response
- ECOG-PS:
-
Eastern Cooperative Oncology Group performance status
- EGFR:
-
Epidermal growth factor receptor
- ICI:
-
Immune checkpoint inhibitor
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- PD:
-
Progressive disease
- PD-1:
-
Programmed death protein 1
- PD-L1:
-
Programmed death ligand 1
- PR:
-
Partial response
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- SD:
-
Stable disease
- SLD:
-
Sum of the longest diameter
- TPS:
-
Tumor proportion score
- TKI:
-
Tyrosine kinase inhibitor
- iUPD:
-
Unconfirmed progressive disease
References
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69(1):7–34. https://doi.org/10.3322/caac.21551
Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA (2008) Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 83(5):584–594. https://doi.org/10.4065/83.5.584
Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Aren Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR (2015) Nivolumab versus Docetaxel in advanced squamous-cell non-small-cell lung Cancer. N Engl J Med 373(2):123–135. https://doi.org/10.1056/NEJMoa1504627
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhaufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crino L, Blumenschein GR Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR (2015) Nivolumab versus Docetaxel in advanced nonsquamous non-small-cell lung Cancer. N Engl J Med 373(17):1627–1639. https://doi.org/10.1056/NEJMoa1507643
Borcoman E, Kanjanapan Y, Champiat S, Kato S, Servois V, Kurzrock R, Goel S, Bedard P, Le Tourneau C (2019) Novel patterns of response under immunotherapy. Ann Oncol 30(3):385–396. https://doi.org/10.1093/annonc/mdz003
Fujimoto D, Yoshioka H, Kataoka Y, Morimoto T, Hata T, Kim YH, Tomii K, Ishida T, Hirabayashi M, Hara S, Ishitoko M, Fukuda Y, Hwang MH, Sakai N, Fukui M, Nakaji H, Morita M, Mio T, Yasuda T, Sugita T, Hirai T (2019) Pseudoprogression in previously treated patients with non-small cell lung Cancer who received Nivolumab monotherapy. J Thorac Oncol 14(3):468–474. https://doi.org/10.1016/j.jtho.2018.10.167
Katz SI, Hammer M, Bagley SJ, Aggarwal C, Bauml JM, Thompson JC, Nachiappan AC, Simone CB 2nd, Langer CJ (2018) Radiologic Pseudoprogression during anti-PD-1 therapy for advanced non-small cell lung Cancer. J Thorac Oncol 13(7):978–986. https://doi.org/10.1016/j.jtho.2018.04.010
Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S, Chaput N, Eggermont A, Marabelle A, Soria JC, Ferte C (2017) Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin Cancer Res 23(8):1920–1928. https://doi.org/10.1158/1078-0432.CCR-16-1741
Kim CG, Kim KH, Pyo KH, Xin CF, Hong MH, Ahn BC, Kim Y, Choi SJ, Yoon HI, Lee JG, Lee CY, Park SY, Park SH, Cho BC, Shim HS, Shin EC, Kim HR (2019) Hyperprogressive disease during PD-1/PD-L1 blockade in patients with non-small-cell lung cancer. Ann Oncol 30:1104–1131. https://doi.org/10.1093/annonc/mdz123
Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbe C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS (2009) Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 15(23):7412–7420. https://doi.org/10.1158/1078-0432.ccr-09-1624
Tabatabai R, Natale R (2018) Immunotherapy and mixed radiographic response in non-small cell lung Cancer. J Cancer Clin 1(1):1005
Tazdait M, Mezquita L, Lahmar J, Ferrara R, Bidault F, Ammari S, Balleyguier C, Planchard D, Gazzah A, Soria JC, Marabelle A, Besse B, Caramella C (2018) Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer 88:38–47. https://doi.org/10.1016/j.ejca.2017.10.017
Nishino M, Dahlberg SE, Adeni AE, Lydon CA, Hatabu H, Janne PA, Hodi FS, Awad MM (2017) Tumor response dynamics of advanced non-small cell lung Cancer patients treated with PD-1 inhibitors: imaging markers for treatment outcome. Clin Cancer Res 23(19):5737–5744. https://doi.org/10.1158/1078-0432.CCR-17-1434
Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P, Mitchell A, Bolejack V, International Association for the Study of Lung Cancer S, Prognostic Factors Committee AB, Participating I, International Association for the Study of Lung Cancer S, Prognostic Factors Committee Advisory B, Participating I (2016) The IASLC lung Cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung Cancer. J Thorac Oncol 11(1):39–51. https://doi.org/10.1016/j.jtho.2015.09.009
Nagai Y, Miyazawa H, Huqun TT, Udagawa K, Kato M, Fukuyama S, Yokote A, Kobayashi K, Kanazawa M, Hagiwara K (2005) Genetic heterogeneity of the epidermal growth factor receptor in non-small cell lung cancer cell lines revealed by a rapid and sensitive detection system, the peptide nucleic acid-locked nucleic acid PCR clamp. Cancer Res 65(16):7276–7282. https://doi.org/10.1158/0008-5472.CAN-05-0331
Vanderlaan PA, Yamaguchi N, Folch E, Boucher DH, Kent MS, Gangadharan SP, Majid A, Goldstein MA, Huberman MS, Kocher ON, Costa DB (2014) Success and failure rates of tumor genotyping techniques in routine pathological samples with non-small-cell lung cancer. Lung Cancer 84(1):39–44. https://doi.org/10.1016/j.lungcan.2014.01.013
Roach C, Zhang N, Corigliano E, Jansson M, Toland G, Ponto G, Dolled-Filhart M, Emancipator K, Stanforth D, Kulangara K (2016) Development of a Companion Diagnostic PD-L1 Immunohistochemistry Assay for Pembrolizumab Therapy in Non–Small-cell Lung Cancer. 24(6):392–397. https://doi.org/10.1097/pai.0000000000000408
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A, Hodi FS, Therasse P, Hoekstra OS, Shankar LK, Wolchok JD, Ballinger M, Caramella C, De Vries EGE (2017) iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 18(3):e143–e152. https://doi.org/10.1016/s1470-2045(17)30074-8
Hong L, Negrao MV, Dibaj SS, Chen R, Reuben A, Bohac JM, Liu X, Skoulidis F, Gay CM, Cascone T, Mitchell KG, Tran HT, Le X, Byers LA, Sepesi B, Altan M, Elamin YY, Fossella FV, Kurie JM, Lu C, Mott FE, Tsao AS, Rinsurongkawong W, Lewis J, Gibbons DL, Glisson BS, Blumenschein GR Jr, Roarty EB, Futreal PA, Wistuba II, Roth JA, Swisher SG, Papadimitrakopoulou VA, Heymach JV, Lee JJ, Simon GR, Zhang J (2020) Programmed death ligand 1 heterogeneity and its impact on benefit from immune checkpoint inhibitors in non-small-cell lung Cancer. J Thorac Oncol S1556-0864(1520):30373–30377. https://doi.org/10.1016/j.jtho.2020.04.026
Lee Y, Kim HY, Lee SH, Lim KY, Lee GK, Yun T, Han JY, Kim HT, Lee JS (2014) Clinical significance of heterogeneity in response to retreatment with epidermal growth factor receptor tyrosine kinase inhibitors in patients with lung cancer acquiring secondary resistance to the drug. Clin Lung Cancer 15(2):145–151. https://doi.org/10.1016/j.cllc.2013.11.008
Kruit WH, van Ojik HH, Brichard VG, Escudier B, Dorval T, Dreno B, Patel P, van Baren N, Avril MF, Piperno S, Khammari A, Stas M, Ritter G, Lethe B, Godelaine D, Brasseur F, Zhang Y, van der Bruggen P, Boon T, Eggermont AM, Marchand M (2005) Phase 1/2 study of subcutaneous and intradermal immunization with a recombinant MAGE-3 protein in patients with detectable metastatic melanoma. Int J Cancer 117(4):596–604. https://doi.org/10.1002/ijc.21264
Jespersen H, Bjursten S, Ny L, Levin M (2018) Checkpoint inhibitor-induced sarcoid reaction mimicking bone metastases. Lancet Oncol 19(6):e327. https://doi.org/10.1016/s1470-2045(18)30252-3
Gkiozos I, Kopitopoulou A, Kalkanis A, Vamvakaris IN, Judson MA, Syrigos KN (2018) Sarcoidosis-like reactions induced by checkpoint inhibitors. J Thorac Oncol 13(8):1076–1082. https://doi.org/10.1016/j.jtho.2018.04.031
Osorio JC, Arbour KC, Le DT, Durham JN, Plodkowski AJ, Halpenny DF, Ginsberg MS, Sawan P, Crompton JG, Yu HA, Namakydoust A, Nabet BY, Chaft JE, Riely GJ, Rizvi H, Diaz LA, Hellmann MD (2019) Lesion-level response dynamics to programmed cell death protein (PD-1) blockade. J Clin Oncol 37(36):3546–3555. https://doi.org/10.1200/jco.19.00709
Remon J, Majem M (2013) EGFR mutation heterogeneity and mixed response to EGFR tyrosine kinase inhibitors of non small cell lung cancer: a clue to overcoming resistance. Transl Lung Cancer Res 2(6):445–448. https://doi.org/10.3978/j.issn.2218-6751.2013.10.14
Shinno Y, Goto Y, Sato J, Morita R, Matsumoto Y, Murakami S, Kanda S, Horinouchi H, Fujiwara Y, Yamamoto N, Ohe Y (2019) Mixed response to osimertinib and the beneficial effects of additional local therapy. Thorac Cancer 10(4):738–743. https://doi.org/10.1111/1759-7714.12991
Dong ZY, Zhai HR, Hou QY, Su J, Liu SY, Yan HH, Li YS, Chen ZY, Zhong WZ, Wu YL (2017) Mixed responses to systemic therapy revealed potential genetic heterogeneity and poor survival in patients with non-small cell lung Cancer. Oncologist 22(1):61–69. https://doi.org/10.1634/theoncologist.2016-0150
Chen ZY, Zhong WZ, Zhang XC, Su J, Yang XN, Chen ZH, Yang JJ, Zhou Q, Yan HH, An SJ, Chen HJ, Jiang BY, Mok TS, Wu YL (2012) EGFR mutation heterogeneity and the mixed response to EGFR tyrosine kinase inhibitors of lung adenocarcinomas. Oncologist 17(7):978–985. https://doi.org/10.1634/theoncologist.2011-0385
Acknowledgements
The authors would like to thank Keiko Sakuragawa and Kanako Masuta for her administrative assistance, and Yukihiro Imai for conducting the pathological analyses. We would like to thank Editage (www.editage.com) for English language editing.
Authors’ information
Yuki Sato: yuki1130sato@gmail.com; Takeshi Morimoto: morimoto@kuhp.kyoto-u.ac.jp; Shigeo Hara: shigeo_hara@kcho.jp; Kazutaka Hosoya: hsyn0917@gmail.com; Kazuma Nagata: knagata@kcho.jp; Atsushi Nakagawa: a.nakagawa@kcho.jp; Ryo Tachikawa: ryotkw@gmail.com; Keisuke Tomii; ktomii@kcho.jp
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted with the approval of the Kobe City Medical Center General Hospital Ethics Committee (No. zn190108).
Consent for publication
Informed consent was not required owing to the retrospective nature of the study.
Competing interests
Dr. Sato has received lecture fees from Ono Pharmaceutical Co., Ltd. (Osaka, Japan). Dr. Morimoto has received manuscript preparation fees and was on an advisory board of Bristol-Myers Squibb K.K. (Tokyo, Japan). Dr. Hosoya has received lecture fees from Ono Pharmaceutical Co., Ltd. (Osaka, Japan). All remaining authors have no conflicts of interest to declare. We wish to confirm that there are no other known conflicts of interest associated with this publication. Further, there was no significant financial support for this work that could have influenced its outcome.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sato, Y., Morimoto, T., Hara, S. et al. Dissociated response and clinical benefit in patients treated with nivolumab monotherapy. Invest New Drugs 39, 1170–1178 (2021). https://doi.org/10.1007/s10637-021-01077-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-021-01077-7