Summary
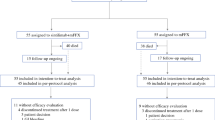
Background Treatment options for pancreatic ductal adenocarcinoma (PDAC) are limited and checkpoint blockade inhibitors have been disappointing in this disease. Pegilodecakin has demonstrated single agent anti-tumor activity in immune-sensitive tumors. Phase 1 and preclinical data indicate synergy of pegilodecakin with 5-FU and platins. We assessed the safety and activity of pegilodecakin+FOLFOX in patients with PDAC. Methods IVY (NCT02009449) was an open-label phase 1b trial in the United States. Here we report on all enrolled patients from cohort C. Heavily pretreated patients were treated with pegilodecakin (self-administered subcutaneously daily at 2.5, 5, or 10 μg/kg) + 5-flurouracil/leucovorin/oxaliplatin (FOLFOX), dosed per manufacturers prescribing information, until tumor progression. Eligible patients had measurable disease per immune-related response criteria (irRC), were ≥ 18 years of age, and had ECOG performance status of 0 or 1. Patients were evaluated for primary(safety) and secondary (tumor response per irRC) endpoints. Results From 5 August 2014–12 July 2016, 39 patients enrolled in cohort C. All patients were evaluable for safety. In this advanced population, regimen had manageable toxicities with no immune-related adverse events (irAEs) greater than grade 1. The most common grade 3/4/5 TEAEs were thrombocytopenia (21[53.8%] of 39) and anemia (17[43.6%] of 39). In evaluable PDAC patients, the best overall response of pegilodecakin+FOLFOX was 3(14%) with CRs in 2(9%) patients. Conclusions Pegilodecakin+FOLFOX had an acceptable tolerability profile in PDAC, with no substantial irAEs seen, and promising efficacy with the combination yielding a 2-year OS of 24% (95% CI 10–42). These data led to the phase 3 study with pegilodecakin+FOLFOX as second-line therapy of PDAC (SEQUOIA).


Similar content being viewed by others
Data availability
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available for request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
References
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67:7–30
Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M, Groupe Tumeurs Digestives of Unicancer, PRODIGE Intergroup (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–1825
Kieler M, Unseld M, Bianconi D, Prager GW (2017) Cross-over comparison and new chemotherapy regimens in metastatic pancreatic cancer. Memo 10:136–140
Zaanan A, Trouilloud I, Markoutsaki T, Gauthier M, Dupont-Gossart AC, Lecomte T, Aparicio T, Artru P, Thirot-Bidault A, Joubert F, Fanica D, Taieb J (2014) FOLFOX as second-line chemotherapy in patients with pretreated metastatic pancreatic cancer from the FIRGEM study. BMC Cancer 14:441
Berk V, Ozdemir N, Ozkan M, Aksoy S, Turan N, Inal A, Balakan O, Yasar N, Unal OU, Benekli M, Durnali A, Colak D, Sonmez OU (2012) XELOX vs. FOLFOX4 as second line chemotherapy in advanced pancreatic cancer. Hepatogastroenterology 59:2635–2639
Chung JW, Jang HW, Chung MJ, Park JY, Park SW, Chung JB, Song SY, Bang S (2013) Folfox4 as a rescue chemotherapy for gemcitabine-refractory pancreatic cancer. Hepatogastroenterology 60:363–367
Wang-Gillam A, Li CP, Bodoky G, Dean A, Shan YS, Jameson G, Macarulla T, Lee KH, Cunningham D, Blanc JF, Hubner RA, Chiu CF, Schwartsmann G, Siveke JT, Braiteh F, Moyo V, Belanger B, Dhindsa N, Bayever E, von Hoff DD, Chen LT, Adoo C, Anderson T, Asselah J, Azambuja A, Bampton C, Barrios CH, Bekaii-Saab T, Bohuslav M, Chang D, Chen JS, Chen YC, Choi HJ, Chung IJ, Chung V, Csoszi T, Cubillo A, DeMarco L, de Wit M, Dragovich T, Edenfield W, Fein LE, Franke F, Fuchs M, Gonzales-Cruz V, Gozza A, Fernando RH, Iaffaioli R, Jakesova J, Kahan Z, Karimi M, Kim JS, Korbenfeld E, Lang I, Lee FC, Lee KD, Lipton L, Ma WW, Mangel L, Mena R, Palmer D, Pant S, Park JO, Piacentini P, Pelzer U, Plazas JG, Prasad C, Rau KM, Raoul JL, Richards D, Ross P, Schlittler L, Smakal M, Stahalova V, Sternberg C, Seufferlein T, Tebbutt N, Vinholes JJ, Wadlow R, Wenczl M, Wong M (2016) Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 387:545–557
Wainberg Z. FK, Lee M-A., Munoz A., Cubilo Gracian A., Lonardi S., Ryoo B-Y., Hung A., Lin Y., Bendell J., Hecht J.R. . Meta-analysis of OS for pancreatic cancer patients receiving 5-FU and oxaliplatin-based therapy after failing first-line gemcitabine-containing therapy. ASCO-GI 2019 Conference 2019
Gong J, Hendifar A, Tuli R et al (2018) Combination systemic therapies with immune checkpoint inhibitors in pancreatic cancer: overcoming resistance to single-agent checkpoint blockade. Clin Transl Med 7:32
Brahmer JR, Tykodi SS, Chow LQ et al (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366:2455–2465
Patnaik A, Kang SP, Rasco D, Papadopoulos KP, Elassaiss-Schaap J, Beeram M, Drengler R, Chen C, Smith L, Espino G, Gergich K, Delgado L, Daud A, Lindia JA, Li XN, Pierce RH, Yearley JH, Wu D, Laterza O, Lehnert M, Iannone R, Tolcher AW (2015) Phase I study of Pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res 21:4286–4293
Zhang J, Wolfgang CL, Zheng L (2018) Precision Immuno-oncology: prospects of individualized immunotherapy for pancreatic Cancer. Cancers (Basel) 10
Johnson BA 3rd, Yarchoan M, Lee V et al (2017) Strategies for increasing pancreatic tumor immunogenicity. Clin Cancer Res 23:1656–1669
Daley D, Zambirinis CP, Seifert L, Akkad N, Mohan N, Werba G, Barilla R, Torres-Hernandez A, Hundeyin M, Mani VRK, Avanzi A, Tippens D, Narayanan R, Jang JE, Newman E, Pillarisetty VG, Dustin ML, Bar-Sagi D, Hajdu C, Miller G (2016) Gammadelta T cells support pancreatic Oncogenesis by restraining alphabeta T cell activation. Cell 166:1485–1499 e1415
Oft M (2014) IL-10: master switch from tumor-promoting inflammation to antitumor immunity. Cancer Immunol Res 2:194–199
Mumm JB, Emmerich J, Zhang X, Chan I, Wu L, Mauze S, Blaisdell S, Basham B, Dai J, Grein J, Sheppard C, Hong K, Cutler C, Turner S, LaFace D, Kleinschek M, Judo M, Ayanoglu G, Langowski J, Gu D, Paporello B, Murphy E, Sriram V, Naravula S, Desai B, Medicherla S, Seghezzi W, McClanahan T, Cannon-Carlson S, Beebe AM, Oft M (2011) IL-10 elicits IFNgamma-dependent tumor immune surveillance. Cancer Cell 20:781–796
Naing A, Papadopoulos KP, Autio KA, Ott PA, Patel MR, Wong DJ, Falchook GS, Pant S, Whiteside M, Rasco DR, Mumm JB, Chan IH, Bendell JC, Bauer TM, Colen RR, Hong DS, van Vlasselaer P, Tannir NM, Oft M, Infante JR (2016) Safety, antitumor activity, and immune activation of Pegylated recombinant human Interleukin-10 (AM0010) in patients with advanced solid tumors. J Clin Oncol 34:3562–3569
Cheeseman SL, Joel SP, Chester JD, Wilson G, Dent JT, Richards FJ, Seymour MT (2002) A 'modified de Gramont' regimen of fluorouracil, alone and with oxaliplatin, for advanced colorectal cancer. Br J Cancer 87:393–399
Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbe C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS (2009) Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clinical cancer research : an official journal of the American Association for Cancer Research 15:7412–7420
Horton B, Spranger S (2018) A tumor cell-intrinsic yin-Yang determining immune evasion. Immunity 49:11–13
Zhao J, Xiao Z, Li T, Chen H, Yuan Y, Wang YA, Hsiao CH, Chow DSL, Overwijk WW, Li C (2018) Stromal modulation reverses primary resistance to immune checkpoint blockade in pancreatic Cancer. ACS Nano 12:9881–9893
Wagner R, Janjigian M, Myers RR (1998) Anti-inflammatory interleukin-10 therapy in CCI neuropathy decreases thermal hyperalgesia, macrophage recruitment, and endoneurial TNF-alpha expression. Pain 74:35–42
Oettle H, Riess H, Stieler JM, Heil G, Schwaner I, Seraphin J, Görner M, Mölle M, Greten TF, Lakner V, Bischoff S, Sinn M, Dörken B, Pelzer U (2014) Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J Clin Oncol 32:2423–2429
Hecht JRNA, Falchook GS et al (2018) Overall survival of PEGylated pegilodecakin with 5-FU/LV and oxaliplatin (FOLFOX) in metastatic pancreatic adenocarcinoma (PDAC). J Clin Oncol 36:4119
Maeda K, Hazama S, Tokuno K, Kan S, Maeda Y, Watanabe Y, Kamei R, Shindo Y, Maeda N, Yoshimura K, Yoshino S, Oka M (2011) Impact of chemotherapy for colorectal cancer on regulatory T-cells and tumor immunity. Anticancer Res 31:4569–4574
Kalanxhi E, Meltzer S, Schou JV, Larsen FO, Dueland S, Flatmark K, Jensen BV, Hole KH, Seierstad T, Redalen KR, Nielsen DL, Ree AH (2018) Systemic immune response induced by oxaliplatin-based neoadjuvant therapy favours survival without metastatic progression in high-risk rectal cancer. Br J Cancer 118:1322–1328
Acknowledgements
Kristi Gruver, employee of Eli Lilly and Company, provided medical writing assistance. Editorial assistance was provided by Antonia Baldo. All writing and editorial assistance was funded by Eli Lilly and Company. We thank all the patients who contributed to this study and to all the staff who worked on this project. This study was a collaboration between researchers from different institutions: MD Anderson Cancer Center, Sarah Cannon Research Institute, University of California Los Angeles (UCLA), START Center for Cancer Care, Memorial Sloan Kettering Cancer Center, University of Oklahoma, and ARMO BioSciences, a wholly owned subsidiary of Eli Lilly and Company.
Funding
The work was supported by ARMO BioSciences, a wholly owned subsidiary of Eli Lilly and Company.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J. Randolph Hecht declares honoraria from ARMO BioSciences a wholly owned subsidiary of Eli Lilly and Company, AstraZeneca, Bristol-Myers Squibb, Gritstone, Halozyme, Ipsen, Merck, Roche; research funding from Abbvie, Advaxis, Amgen, ARMO BioSciences a wholly owned subsidiary of Eli Lilly and Company, Astellas Pharma, Forty Seven, Halozyme, Immunomedics, Eli Lilly and Company, Merck, Novartis; and travel accommodations and expenses from Advaxis, Eli Lilly and Company. Kyriakos P. Papadopoulos declares research funding from Abbvie, Amgen, ArQule, ARMO Biosciences a wholly owned subsidiary of Eli Lilly and Company, ADC Therapeutics, Anheart, 3D Medicines, Basilia, Bayer, Calithera Biosciences, Daiichi Sankyo, EMD Serono, F-star, Incyte, Jounce Therapeutics, Linnaeus, Mabspace Biosciences, Merck, Mirati Therapeutics, MedImmune, Mersana, Peloton Therapeutics, Regeneron, Syros Pharmaceuticals and Tempest Therapeutics; advisory board fees from Arqule, Basilia, Bayer. Gerald S. Falchook declares consulting or advisory role with EMD Serono, Fujifilm; speakers’ bureau with Total Health Conferencing, Rocky Mountain Oncology Society; research funding from 3-V Biosciences, Abbisko, Abbvie, ADC Therapeutics, Aileron Therapeutics, American Society of Clinical Oncology, Amgen, ARMO BioSciences a wholly owned subsidiary of Eli Lilly and Company, AstraZeneca, BeiGene, Bioatla, Bioinvent, Biothera, Bicycle, Celgene, Celldex, Ciclomed, Curegenix, Curis, Cyteir, Daiichi, DelMar Pharmaceuticals, eFFECTOR Therapeutics, Eli Lilly and Company, EMD Serono, Epizyme, Exelixis, Fujifilm, Genmab, GlaxoSmithKline, Hutchison MediPharma, Ignyta, Incyte, Jacobio, Jounce Therapeutics, Koltan Pharmaceuticals, Loxo, MedImmune, Merck, Millenium, miRNA Therapeutics, National Institutes of Health, Novartis, OncoMed, Oncorus, Oncothyreon, Poseida, Precision Oncology, Prelude, Regeneron, Rgenix, Ribon Therapeutics, Sapience, Strategia Therapeutics, Synthorx, Syndax, Taiho Pharmaceutical, Takeda, Tarveda Therapeutics, Tesaro, Tocagen, Turning Point Therapeutics, U.T. MD Anderson Cancer Center, Vegenics, Xencor; patents royalties and other intellectual property with Wolters Kluwer; and travel accommodations and expenses from Bristol-Myers Squibb, EMD Serono, Fujifilm, Millenium, Sarah Cannon Research Institute. Manish R. Patel declares honoraria from Bayer, Genentech, Janssen Oncology, Pfizer, Pharmacyclics; consulting of advisory role with Pfizer/EMD Serono, Pharmacyclics/Janssen; speakers’ bureau with Celgene, Exelixis, Genentech/Roche, Taiho Pharmaceutical; research funding from Acerta Pharma, ADC Therapeutics, Agenus, Aileron Therapeutics, AstraZeneca, Bicycle Therapeutics, BioNTech AG, Boehringer Ingelheim, Calithera Biosciences, Celgene, Checkpoint Therapeutics, CicloMed, Clovis Oncology, Curis, Cyteir Therapeutics, Daiichi Sankyo, eFFECTOR Therapeutics, EMD Serono, Evelo Therapeutics, FORMA Therapeutics, Genentech/Roche, Gilead Sciences, GlaxoSmithKline, H3 Biomedicine, Hengrui Therapeutics, Hutchison MediPharma, Ignyta, Incyte, Jacobio, Janssen, Jounce Therapeutics, Klus Pharma, Kymab, Eli Lilly and Company, Loxo, LSK Biopartners, Lycera, Macrogenics, Merck, Millenium, Mirati Therapeutics, Moderna Therapeutics, Pfizer, Phoenix Molecular Designs, Placon, Portola Pharmaceuticals, Prelude Therapeutics, Puget Sound Biotherapeutics, QiLu Pharmaceuticals, Stemline Therapeutics, Syndax, Synthorx, Taiho Pharmaceutical, Takeda, Tesaro, TopAlliance BioSciences Inc., Vedanta Biosciences, Verastem, Vigeo, Xencor. Jeffrey R. Infante declares employment with Janssen Research & Development with stock and other ownership interests with Johnson & Johnson. Raid Aljumaily declares consulting or advisory role with AstraZeneca, Regeneron; research funding from Abbvie, Alliance Foundation Trials LLC, Array BioPharma, AstraZeneca, Baxalta, Boehringer Ingelheim, Boston Biomedical, Bristol-Myers Squibb, Checkpoint Therapeutics Inc., EMD Serono, F. Hoffman-La Roche AG, G1 Therapeutics, Genentech, GlaxoSmithKline, Huntsman Cancer Institute, Eli Lilly and Company, MedImmune LLC, Merck Co, Novartis, Peloton Therapeutics Inc., Pfizer, Regeneron, Syneos Health, Tesaro. Karen A. Autio declares research funding from Amgen, ARMO BioSciences a wholly owned subsidiary of Eli Lilly and Company, AstraZeneca, CytomX Therapeutics, GlaxoSmithKline, Merck, Pfizer, Tizona Therapeutics Inc. Zev A. Wainberg declares consulting or advisory role with AstraZeneca, Bayer, Eli Lilly and Company, Merck, Novartis; research funding from Novartis, Ipsen, Plexxikon. Todd M. Bauer declares research funding from Daiichi Sankyo, Medpacto, Incyte, Mirati Therapeutics, MedImmune, Abbvie, AstraZeneca, MabVax, Stemline Therapeutics, Merck, Eli Lilly and Company, GlaxoSmithKline, Novartis, Genentech, Deciphera, Merrimack, Immunogen, Millennium, Phosplatin Therapeutics, Calithera Biosciences, Kolltan Pharmaceuticals, Principa Biopharma, Peleton, Immunocore, Roche, Aileron Therapeutics, Bristol-Myers Squibb, Amgen, Onyx, Sanofi, Boehringer-Ingelheim, Astellas Pharma, Five Prime Therapeutics, Jacobio, Top Alliance BioScience, Janssen, Clovis Oncology, Takeda, Karyopharm Therapeutics, Foundation Medicine, ARMO Biosciences a wholly owned subsidiary of Eli Lilly and Company, Leap Therapeutics; grants and non-financial support and consulting services and/or personal fees from Ignyta, Moderna Therapeutics, Pfizer, Loxo, Bayer, Guardant Health; and personal fees from Exelesis. Milind Javle declares consulting or advisory role with Incyte, Mundi, Oncosil, QED; other relationship with Bayer, BeiGene, Incyte, Merck, Merck Serono, Novartis, Pieris Pharmaceuticals, QED Therapeutics, Rafael Pharmaceuticals, Seattle Genetics. Shubham Pant declares honoraria from 4D Pharma; consulting or advisory role with TYME, Xencor, Ipsen; and research funding from ArQule, Bristol-Myers Squibb, Five Prime Therapeutics, GlaxoSmithKline, Ipsen, Eli Lilly and Company, Mirati Therapeutics, Novartis, Onco Response, RedHill BioPharma, Rgenix, Sanofi/Aventis, Xencor. Johanna Bendell declares research funding Gilead, Genentech/Roche, Bristol-Myers Squibb, Five Prime, Eli Lilly and Company, Merck, MedImmune, Celgene, EMD Serono, Taiho, Macrogenics, GSK, Novartis, OncoMed, LEAP, TG Therapeutics, AstraZeneca, BI, Daiichi Sankyo, Bayer, Incyte, Apexigen, Koltan, SynDevRex, Forty Seven, Abbvie, Array, Onyx, Sanofi, Takeda, Eisai, Celldex, Agios, Cytomx, Nektar, ARMO BioSciences a wholly owned subsidiary of Eli Lilly and Company, Boston Biomedical, Ipsen, Merrimack, Tarveda, Tyrogenex, Oncogenex, Marshall Edwards, Pieris, Mersana, Calithera, Blueprint, Evelo, FORMA, Merus, Jacobio, Effector, Novocare, Arrys, Tracon, Sierra, Innate, Arch Oncology, Prelude Therapeutics, Unum Therapeutics, Vyriad, Harpoon, ADC, Amgen, Pfizer, Millenium, Imclone, Acerta Pharma, Rgenix, Bellicum, Gossamer Bio, Arcus Bio, Seattle Genetics, Tempest Tx, Shattuck Labs, Synthorx Inc., Revolution Medicines Inc., Bicycle Therapeutics, Zymeworks, Relay Therapeutics, Atlas Medx, Scholar Rock, NGM Biopharma; consulting services with Gilead, Genentech/Roche, Bristol-Myers Squibb, Five Prime, Eli Lilly and Company, Merck, MedImmune, Celgene, Taiho, Macrogenics, GSK, Novartis, OncoMed, LEAP, TG Therapeutics, AstraZeneca, BI, Daiichi Sankyo, Bayer, Incyte, Apexigen, Ipsen, Merrimack, Oncogenex, Evelo, FORMA, Innate, Arch Oncology, Prelude Therapeutics, Bicycle Therapeutics, Relay Therapeutics, Phoenix Bio, Cyteir, Molecular Partners, Torque, Tizona, Janssen, Tolero, TD2 (Translational Drug Development), Moderna Therapeutics, Tanabe Research Laboratories, Beigene, Continuum Clinical, Amgen, Evelo, Piper Biotech, Samsung Bioepios, ARMO BioSciences a wholly owned subsidiary of Eli Lilly and Company, Agios, Sanofi, Array. Joseph Leveque declares previous employment with stock holdings from ARMO BioSciences a wholly owned subsidiary of Eli Lilly and Company and Synthorx and current employment with stock holdings from Mirati Therapeutics (serving as CMO). Navneet Ratti, Peter VanVlasselaer, Rakesh Verma, and Martin Oft declare former employment with ARMO BioSciences a wholly owned subsidiary of Eli Lilly and Company with stock holdings. Annie Hung and Sujata Rao declare former employment with ARMO BioSciences a wholly owned subsidiary of Eli Lilly and Company and current employment of Eli Lilly and Company with stock holdings. Aung Naing declares consulting or advisory role with CytomX Therapeutics, Novartis, Kymab, Genome; research funding with Amplimmune, ARMO BioSciences a wholly owned subsidiary of Eli Lilly and Company, Atterocor, Bristol-Myers Squibb, Calithera BioSciences, CytomX Therapeutics, EMD Serono, Healios Onc Nutrition, Immune Deficiency Foundation (Spouse), Incyte, Karyopharm Therapeutics, Kymab, Eli Lilly and Company, MedImmune, Merck, NCI, Neon Therapeutics, Novartis, Pfizer, PsiOxus Therapeutics, Regeneron, TopAlliance BioSciences Inc.; travel, accommodations, and expenses from ARMO BioSciences a wholly owned subsidiary of Eli Lilly and Company.
Ethical approval
All procedures performed in the study involving human participants were in accordance with Good Clinical Practice Guidelines (GCP), the US Code of Federal Regulations governing the protection of human patients (21 CFR 50), International Conference on Harmonization (ICH) guidelines, local ethical requirements consistent with the current version of the Declaration of Helsinki, and the Institutional Review Board or Independent Ethics Committees (IEC; 21 CFR 56).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 211 kb)
Rights and permissions
About this article
Cite this article
Hecht, J.R., Papadopoulos, K.P., Falchook, G.S. et al. Immunologic and tumor responses of pegilodecakin with 5-FU/LV and oxaliplatin (FOLFOX) in pancreatic ductal adenocarcinoma (PDAC). Invest New Drugs 39, 182–192 (2021). https://doi.org/10.1007/s10637-020-01000-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-020-01000-6