Summary
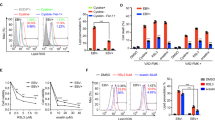
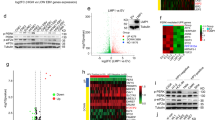
Recent studies revealed an unexpected role of the neurotrophin receptor pathway, BDNF/TrkB signaling, in cancer metastasis and anoikis (i.e. detachment-induced cell death). Survival of cancer cells in detached state (known as anoikis-resistance) is known to be pre-requisite for metastasis. Nasopharyngeal carcinoma (NPC), an endemic head and neck cancer in Southeast Asia, is highly invasive, metastatic, and etiologically associated with Epstein-Barr virus (EBV, an oncovirus) infection. Mechanistic studies on the invasive/metastatic nature of NPC can facilitate the development of anti-metastatic therapy in NPC. Thus far, the role of BDNF/TrkB signaling in virus-associated human cancer is unclear. Here, using multiple cell line models of NPC with EBV-association (HONE-1-EBV, HK1-LMP1 and C666-1), we investigated the potential involvement of BDNF/TrkB signaling in cellular migration and anoikis-resistant characteristics of NPC. We found that all three EBV-associated NPC cell lines tested were intrinsically anoikis-resistant (i.e. survived in detached state) and expressed both BDNF and TrkB. BDNF stimulation induced cellular migration, but not proliferation of these cells. Further, we examined if pharmacologic targeting of anoikis-resistance of NPC cells can be achievable by a proof-of-concept Trk inhibitor, K252a, in these EBV-associated NPC models. Our results demonstrated that K252a, was able to attenuate BDNF-induced migration and proliferation of NPC cells. More importantly, we demonstrated for the first time that K252a harbored potent anoikis-sensitization activity (i.e. sensitizing cancer cells to detachment-induced cell death) against EBV-associated human cancer cells, namely NPC cells. This proof-of-concept study demonstrated that K252a, a Trk inhibitor, can potentially be used as an anoikis-sensitizing agent in NPC.




Similar content being viewed by others
Abbreviations
- NPC:
-
Nasopharyngeal Carcinoma
- EBV:
-
Epstein-Barr virus
- LMP1:
-
Latent Membrane Protein 1
- BDNF:
-
Brain-derived neurotrophic factor
- TrkB:
-
Tropomyosin-related kinase B
References
Raab-Traub N (2002) Epstein-Barr virus in the pathogenesis of NPC. Semin Cancer Biol 12(6):431–441
Niedobitek G (1999) The Epstein-Barr virus: a group 1 carcinogen? Virchows Arch 435(2):79–86
Chan AT, Teo PM, Johnson PJ (2002) Nasopharyngeal carcinoma. Ann Oncol 13(7):1007–1015
Anderson KE, Mack TM, Silverman DT (2006) Cancer of the pancreas. In: Schottenfeld D, Fraumeni JF Jr (eds) Cancer epidemiology and prevention, 3rd edn. Oxford University Press, New York, NY, pp 721–762
Fandi A, Altun M, Azli N et al (1994) Nasopharyngeal cancer: epidemiology, staging, and treatment. Semin Oncol 21(3):382–397
Burt RD, Vaughan TL, McKnight B (1992) Descriptive epidemiology and survival analysis of nasopharyngeal carcinoma in the United States. Int J Cancer 52(4):549–556
Yeung WM, Zong YS, Chiu CT et al (1993) Epstein-Barr virus carriage by nasopharyngeal carcinoma in situ. Int J Cancer 53(5):746–750
Dickens P, Srivastava G, Loke SL et al (1992) Epstein-Barr virus DNA in nasopharyngeal carcinomas from Chinese patients in Hong Kong. J Clin Pathol 45(5):396–397
Klein G, Giovanella BC, Lindahl T et al (1974) Direct evidence for the presence of Epstein-Barr virus DNA and nuclear antigen in malignant epithelial cells from patients with poorly differentiated carcinoma of the nasopharynx. Proc Natl Acad Sci U S A 71(12):4737–4741
Lui VW, Wong EY, Ho Y et al (2009) STAT3 activation contributes directly to Epstein-Barr virus-mediated invasiveness of nasopharyngeal cancer cells in vitro. Int J Cancer 125(8):1884–1893
Chang SH, Chang HC, Hung WC (2008) Transcriptional repression of tissue inhibitor of metalloproteinase-3 by Epstein-Barr virus latent membrane protein 1 enhances invasiveness of nasopharyngeal carcinoma cells. Oral Oncol 44(9):891–897
Tsuji A, Wakisaka N, Kondo S et al (2008) Induction of receptor for advanced glycation end products by EBV latent membrane protein 1 and its correlation with angiogenesis and cervical lymph node metastasis in nasopharyngeal carcinoma. Clin Cancer Res 14(17):5368–5375
Ho CH, Chen CL, Li WY et al (2009) Decoy receptor 3, upregulated by Epstein-Barr virus latent membrane protein 1, enhances nasopharyngeal carcinoma cell migration and invasion. Carcinogenesis 30(8):1443–1451
Agulnik M, Epstein JB (2008) Nasopharyngeal carcinoma: current management, future directions and dental implications. Oral Oncol 44(7):617–627
Frisch SM, Francis H (1994) Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol 124(4):619–626
Wang X-C, Wu Y-P, Ye B et al (2009) Suppression of anoikis by SKP2 amplification and overexpression promotes metastasis of esophageal squamous cell carcinoma. Mol Canc Res 7(1):12–22
Zhang X, Su L, Pirani A et al (2006) Understanding metastatic SCCHN cells from unique genotypes to phenotypes with the aid of an animal model and DNA microarray analysis. Clin Exp Metastasis 23(3):209
Duxbury MS, Ito H, Zinner MJ et al (2004) Focal adhesion kinase gene silencing promotes anoikis and suppresses metastasis of human pancreatic adenocarcinoma cells. Surgery 135(5):555
Duxbury MS, Ito H, Zinner MJ, et al. CEACAM6 gene silencing impairs anoikis resistance and in vivo metastatic ability of pancreatic adenocarcinoma cells. Oncogene 23(2):465
Swan EA, Jasser SA, Holsinger FC et al (2003) Acquisition of anoikis resistance is a critical step in the progression of oral tongue cancer. Oral Oncol 39(7):648
Zhu Z, Sanchez-Sweatman O, Huang X et al (2001) Anoikis and metastatic potential of cloudman S91 melanoma cells. Cancer Res 61(4):1707–1716
Geiger TR, Peeper DS (2007) Critical role for TrkB kinase function in anoikis suppression, tumorigenesis, and metastasis. Cancer Res 67(13):6221–6229
Geiger TR, Peeper DS (2005) The neurotrophic receptor TrkB in anoikis resistance and metastasis: a perspective. Cancer Res 65(16):7033–7036
Douma S, Van Laar T, Zevenhoven J et al (2004) Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature 430(7003):1034–1039
Zhang Z, Han L, Liu Y et al. (2008) Up-regulation of tropomyosin related kinase B contributes to resistance to detachment-induced apoptosis in hepatoma multicellular aggregations. Mol Biol Rep
Zhang Z, Cao L, Li J et al (2008) Acquisition of anoikis resistance reveals a synoikis-like survival style in BEL7402 hepatoma cells. Cancer Lett 267(1):106–115
Yu X, Liu L, Cai B et al (2008) Suppression of anoikis by the neurotrophic receptor TrkB in human ovarian cancer. Cancer Science 99(3):543–552
Sclabas GM, Fujioka S, Schmidt C et al (2005) Overexpression of tropomysin-related kinase B in metastatic human pancreatic cancer cells. Clin Cancer Res 11(2):440–449
Martin-Zanca D, Hughes SH, Barbacid M (1986) A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. Nature 319(6056):743–748
Katzav S, Martin-Zanca D, Barbacid M et al (1989) The trk oncogene abrogates growth factor requirements and transforms hematopoietic cells. Oncogene 4(9):1129–1135
Mawji IA, Simpson CD, Gronda M et al (2007) A chemical screen identifies anisomycin as an anoikis sensitizer that functions by decreasing FLIP protein synthesis. Cancer Res 67(17):8307–8315
Evans AE, Kisselbach KD, Yamashiro DJ et al (1999) Antitumor activity of CEP-751 (KT-6587) on human neuroblastoma and medulloblastoma xenografts. Clin Cancer Res 5(11):3594–3602
Wang Y, Hagel C, Hamel W et al (1998) Trk A, B, and C are commonly expressed in human astrocytes and astrocytic gliomas but not by human oligodendrocytes and oligodendroglioma. Acta Neuropathol 96(4):357–364
Wadhwa S, Nag TC, Jindal A et al (2003) Expression of the neurotrophin receptors Trk A and Trk B in adult human astrocytoma and glioblastoma. J Biosci 28(2):181–188
Yang ZF, Ho DW, Lam CT et al (2005) Identification of brain-derived neurotrophic factor as a novel functional protein in hepatocellular carcinoma. Cancer Res 65(1):219–225
Kupferman ME, Jiffar T, El-Naggar A et al (2010) TrkB induces EMT and has a key role in invasion of head and neck squamous cell carcinoma. Oncogene 29(14):2047–2059
Zhao W, Wen W, Zhang Z et al (2007) Expression and significance of tyrosine kinase receptors B in nasopharyngeal carcinoma patients. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 21(11):497–500
Anderson RA, Robinson LL, Brooks J et al (2002) Neurotropins and their receptors are expressed in the human fetal ovary. J Clin Endocrinol Metab 87(2):890–897
Hong B, Lui VW, Hui EP et al. (2009) Hypoxia-targeting by tirapazamine (TPZ) induces preferential growth inhibition of nasopharyngeal carcinoma cells with Chk1/2 activation. Invest New Drugs
Lui VW, Boehm AL, Koppikar P et al (2007) Antiproliferative mechanisms of a transcription factor decoy targeting signal transducer and activator of transcription (STAT) 3: the role of STAT1. Mol Pharmacol 71(5):1435–1443
Lui VW, Thomas SM, Zhang Q et al (2003) Mitogenic effects of gastrin-releasing peptide in head and neck squamous cancer cells are mediated by activation of the epidermal growth factor receptor. Oncogene 22(40):6183–6193
Lui VW, Yau DM, Wong EY et al (2009) Cucurbitacin I elicits anoikis sensitization, inhibits cellular invasion and in vivo tumor formation ability of nasopharyngeal carcinoma cells. Carcinogenesis 30(12):2085–2094
Diaz-Montero CM, Wygant JN, McIntyre BW (2006) PI3-K/Akt-mediated anoikis resistance of human osteosarcoma cells requires Src activation. Eur J Cancer 42(10):1491–1500
Akinaga S, Ashizawa T, Gomi K et al (1992) Antitumor effect of KT6124, a novel derivative of protein kinase inhibitor K-252a, and its mechanism of action. Cancer Chemother Pharmacol 29(4):266–272
Festuccia C, Gravina GL, Muzi P et al (2007) In vitro and in vivo effects of bicalutamide on the expression of TrkA and P75 neurotrophin receptors in prostate carcinoma. Prostate 67(12):1255–1264
Perez-Pinera P, Hernandez T, Garcia-Suarez O et al (2007) The Trk tyrosine kinase inhibitor K252a regulates growth of lung adenocarcinomas. Mol Cell Biochem 295(1–2):19–26
Miknyoczki SJ, Chang H, Klein-Szanto A et al (1999) The Trk tyrosine kinase inhibitor CEP-701 (KT-5555) exhibits significant antitumor efficacy in preclinical xenograft models of human pancreatic ductal adenocarcinoma. Clin Cancer Res 5(8):2205–2212
Miknyoczki SJ, Dionne CA, Klein-Szanto AJ et al (1999) The novel Trk receptor tyrosine kinase inhibitor CEP-701 (KT-5555) exhibits antitumor efficacy against human pancreatic carcinoma (Panc1) xenograft growth and in vivo invasiveness. Ann N Y Acad Sci 880:252–262
Morotti A, Mila S, Accornero P et al (2002) K252a inhibits the oncogenic properties of Met, the HGF receptor. Oncogene 21(32):4885–4893
Takai N, Ueda T, Nishida M et al (2005) K252a inhibits proliferation of ovarian cancer cells by upregulating p21WAF1. Oncol Rep 14(1):141–143
Takai N, Ueda T, Nishida M et al (2008) K252a is highly effective in suppressing the growth of human endometrial cancer cells, but has little effect on normal human endometrial epithelial cells. Oncol Rep 19(3):749–753
Marshall JL, Kindler H, Deeken J et al (2005) Phase I trial of orally administered CEP-701, a novel neurotrophin receptor-linked tyrosine kinase inhibitor. Invest New Drugs 23(1):31–37
Undevia SD, Vogelzang NJ, Mauer AM et al (2004) Phase I clinical trial of CEP-2563 dihydrochloride, a receptor tyrosine kinase inhibitor, in patients with refractory solid tumors. Invest New Drugs 22(4):449–458
Strock CJ, Park JI, Rosen M et al (2003) CEP-701 and CEP-751 inhibit constitutively activated RET tyrosine kinase activity and block medullary thyroid carcinoma cell growth. Cancer Res 63(17):5559–5563
Smith BD, Levis M, Beran M et al (2004) Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood 103(10):3669–3676
Thress K, Macintyre T, Wang H et al (2009) Identification and preclinical characterization of AZ-23, a novel, selective, and orally bioavailable inhibitor of the Trk kinase pathway. Mol Cancer Ther 8(7):1818–1827
Cazorla M, Jouvenceau A, Rose C et al (2010) Cyclotraxin-B, the first highly potent and selective TrkB inhibitor, has anxiolytic properties in mice. PLoS One 5(3):e9777
Acknowledgement
This study was supported by Direct Grant for Research, The Chinese University of Hong Kong (2008.1.101 to YKN). Result of this study was presented in part in the poster session at the American Association of Cancer Research (AACR) annual meeting, Colorado, USA, 2009.
Conflict of Interest Statement
All authors have no financial/commercial conflicts of interest regarding the study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.

Supplementary Fig. 1
Exogenous BDNF induced TrkB expression in C666-1. C666-1 cells were treated with either serum free medium with vehicle (H2O) or BDNF (50 ng/ml) for 48 h. The expression level of TrkB was determined by Western blotting. Similar results were obtained in 3 independent experiments. (JPEG 9 kb)

Supplementary Fig. 2
K252a inhibited TrkB phosphorylation in an EBV-associated NPC cell line with high expression level of TrkB. HONE-1-EBV cells were treated with either vehicle (DMSO), 300 nM or 3 μM of K252a for 8 h. Cell lysates were then subjected to immunoprecipitation with TrkB antibody. Expression of phosphorylated TrkB was then detected by immunoblotting with anti-phosphorylated antibody (PY20: BD Transduction Lab, Lexington, KY). Similar results were observed in 3 independent experiments. (JPEG 13 kb)
Rights and permissions
About this article
Cite this article
Ng, YK., Wong, E.Y.L., Lau, C.P.Y. et al. K252a induces anoikis-sensitization with suppression of cellular migration in Epstein-Barr Virus (EBV)—associated nasopharyngeal carcinoma cells. Invest New Drugs 30, 48–58 (2012). https://doi.org/10.1007/s10637-010-9513-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-010-9513-4