Abstract
Background
Glutamate-rich WD repeat containing 1 (GRWD1) is over-expressed in a variety of malignant tumors and is considered to be a potential oncogene. However, its mechanism of action in gastric cancer (GC) is still unclear.
Methods
Data analysis, Immunohistochemistry, and Western Blot (WB) were performed to verify the expression of GRWD1 in GC and para-cancerous tissues. The association between GRWD1 expression and tumor size, tissue differentiation, lymph node metastasis, TNM stage, and prognosis was analyzed according to the high and low expression levels of GRWD1. The relationship between GRWD1 and Notch pathway was verified by data analysis and WB. The effects of GRWD1 on the proliferation, migration, and invasion of GC cells were verified by cell proliferation, migration, and invasion assays. We confirmed that the high expression of GRWD1 promoted the proliferation of GC cells in vivo through the tumor formation assay in nude mice.
Results
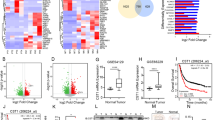
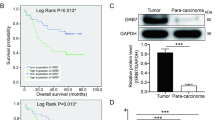
The expression of GRWD1 was higher in GC tissues than in para-cancerous tissues, and its expression was positively correlated with tumor size, lymph node metastasis, and TNM stage, but negatively correlated with differentiation grade and prognosis. GRWD1 over-expression increased ADAM metallopeptidase domain 17 (ADAM17) expression and promoted Notch1 intracellular domain (NICD) release to promote GC cell proliferation, migration, and invasion in vitro. Results from animal studies have shown that high GRWD1 expression could promote GC cell proliferation in vivo by activating the Notch signaling pathway.
Conclusion
GRWD1 promotes GC progression through ADAM17-dependent Notch signaling, and GRWD1 may be a novel tumor marker and therapeutic target.









Similar content being viewed by others
References
Torre LA, Siegel RL, Ward EM, Jemal A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiology, Biomarkers & Prevention. 2016;25:16–27.
Ferlay J, Colombet M, Soerjomataram I et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–1953.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA:A Cancer Journal for Clinicians. 2015; 65: 87–108.
Feng F, Tian Y, Xu G, et al. Diagnostic and prognostic value of CEA, CA19–9, AFP and CA125 for early gastric cancer. Bmc Cancer. 2017; 17.
Takafuji T, Kayama K, Sugimoto N, Fujita M. GRWD1, a new player among oncogenesis-related ribosomal/nucleolar proteins. Cell Cycle. 2017;16:1397–1403.
van Nocker S, Ludwig P. The WD-repeat protein superfamily in Arabidopsis: conservation and divergence in structure and function. Bmc Genomics. 2003;4:50.
Sugimoto N, Maehara K, Yoshida K et al. Cdt1-binding protein GRWD1 is a novel histone-binding protein that facilitates MCM loading through its influence on chromatin architecture. Nucleic Acids Res. 2015;43:5898–5911.
Wu Y, Wu X, Li Y et al. Comprehensive Analysis of Glutamate-Rich WD Repeat-Containing Protein 1 and Its Potential Clinical Significance for Pancancer. Biomed Res Int. 2021;2021:1–16.
Kayama K, Watanabe S, Takafuji T et al. GRWD1 negatively regulates p53 via the RPL11-MDM2 pathway and promotes tumorigenesis. Embo Rep. 2017;18:123–137.
Killian A, Sarafan-Vasseur N, Sesboüé R et al. Contribution of theBOP1 gene, located on 8q24, to colorectal tumorigenesis. Genes, Chromosomes and Cancer. 2006;45:874–881.
Zhou X, Shang J, Liu X et al. Clinical Significance and Oncogenic Activity of GRWD1 Overexpression in the Development of Colon Carcinoma. Onco Targets Ther. 2021;14:1565–1580.
Wang Q, Ren H, Xu Y et al. GRWD1 promotes cell proliferation and migration in non-small cell lung cancer by activating the Notch pathway. Exp Cell Res. 2020;387:111806.
Yao L, Tian F. GRWD1 affects the proliferation, apoptosis, invasion and migration of triple negative breast cancer through the Notch signaling pathway. Exp Ther Med. 2022;24:473.
Fabbri G, Rasi S, Rossi D et al. Analysis of the chronic lymphocytic leukemia coding genome: role of NOTCH1 mutational activation. J Exp Med. 2011;208:1389–1401.
Mollen EWJ, Ient J, Tjan-Heijnen VCG, et al.. Moving Breast Cancer Therapy up a Notch. Front Oncol. 2018; 8.
Robinson DR, Kalyana-Sundaram S, Wu Y et al. Functionally recurrent rearrangements of the MAST kinase and Notch gene families in breast cancer. Nat Med. 2011;17:1646–1651.
Lim JS, Ibaseta A, Fischer MM et al. Intratumoural heterogeneity generated by Notch signalling promotes small-cell lung cancer. Nature. 2017;545:360–364.
Andersson ER, Lendahl U. Therapeutic modulation of Notch signalling — are we there yet? Nat Rev Drug Discov. 2014;13:357–378.
Niessen K, Fu Y, Chang L, Hoodless PA, McFadden D, Karsan A. Slug is a direct Notch target required for initiation of cardiac cushion cellularization. J Cell Biol. 2008;182:315–325.
Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233.
Kalantari E, Saeidi H, Kia NS et al. Effect of DAPT, a gamma secretase inhibitor, on tumor angiogenesis in control mice. Adv Biomed Res. 2013;2:83.
Liu X, Xu Q, Xie W, Wang M. DAPT suppresses the proliferation of human glioma cell line SHG-44. Asian Pac j Trop Med. 2014;7:552–556.
Ichikawa MK, Saitoh M. Direct and indirect roles of GRWD1 in the inactivation of p53 in cancer. The Journal of Biochemistry. 2022;171:601–603.
Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell. 2009;137:413–431.
Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nature reviews. Molecular Cell Biology. 2008;9:402–412.
Levine AJ, Oren M. The first 30 years of p53: growing ever more complex. Nat Rev Cancer. 2009;9:749–758.
Watanabe S, Fujiyama H, Takafuji T, et al.. Glutamate-rich WD40 repeat containing 1 regulates ribosomal protein L23 levels via the ubiquitin-proteasome system. J Cell Sci. 2018.
Wang H, Zang C, Liu XS, Aster JC. The Role of Notch Receptors in Transcriptional Regulation. J Cell Physiol. 2015;230:982–988.
Wang R, Li Y, Tsung A, et al.. iNOS promotes CD24+ CD133+ liver cancer stem cell phenotype through a TACE/ADAM17-dependent Notch signaling pathway. Proceedings of the National Academy of Sciences. 2018; 115.
Delwig A, Rand MD. Kuz and TACE can activate Notch independent of ligand. Cell Mol Life Sci. 2008;65:2232–2243.
Katoh M, Katoh M. WNT antagonist, DKK2, is a Notch signaling target in intestinal stem cells: augmentation of a negative regulation system for canonical WNT signaling pathway by the Notch-DKK2 signaling loop in primates. Int J Mol Med. 2007;19:197.
Xu Y, Ren H, Jiang J et al. KIAA0247 inhibits growth, migration, invasion of non-small-cell lung cancer through regulating the Notch pathway. Cancer Sci. 2018;109:1055–1065.
Wang Y, Zhong Y, Hou T, et al.. PM2.5 induces EMT and promotes CSC properties by activating Notch pathway in vivo and vitro. Ecotox Environ Safe. 2019; 178: 159–67.
Zou Y, Yang R, Huang M, et al.. NOTCH2 negatively regulates metastasis and epithelial-Mesenchymal transition via TRAF6/AKT in nasopharyngeal carcinoma. J Exp Clin Canc Res. 2019; 38.
Acknowledgments
Thanks to the help of all members of Jingtao Lu Laboratory
Funding
This study was funded by the Key Research and Development Project of Anhui Provincial Department of Science and Technology (202004j07020036), Annual scientific research projects in Colleges and Universities of Anhui Provincial Department of Education (2022AH050779), and the Wu Jieping Medical Fund (320.6750.19092-19).
Author information
Authors and Affiliations
Contributions
Ding Huiming designed the study. Ding Huiming and Feng Zhenyou carried out data statistics, experimental verifi cation, and sorted out the data results. Hu Kongwang provided research ideas, participated in the research design, and determined the final research scheme. Hu Kongwang and Ding Huiming prepared the manuscript, and all authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ding, H., Feng, Z. & Hu, K. GRWD1 Over-Expression Promotes Gastric Cancer Progression by Activating Notch Signaling Pathway via Up-Regulation of ADAM17. Dig Dis Sci 69, 821–834 (2024). https://doi.org/10.1007/s10620-023-08208-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-023-08208-5