Abstract
Background
Pancreatic enzyme replacement therapy (PERT) is the standard treatment for exocrine pancreatic insufficiency (EPI). However, many individuals are inadequately treated, with gaps in clinical dosing, guidelines, and tools to aid individual titration.
Methods
A systematic review identified research and guidelines on PERT dosing recommendations across conditions, systematically reviewing and synthesizing total PERT intake, meal/snack guidelines, and changes over time to provide an up-to-date look at the most common doses used in studies and guidelines.
Results
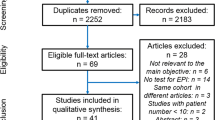
This review of 257 articles found wide variability in PERT dosing guidelines within and across conditions. Many patients with EPI are underdosed, with guidelines differing globally and by disease type, and clinician prescribing may also play a role. The most common dosing guidelines focus on starting doses at 40,000–50,000 units of lipase/meal with increases of up to two to three times this amount before pursuing additive therapies. Guidelines and studies typically focus only on fat digestion, and comparison by total daily dose shows underdosing is common. Most PERT studies are on safety and efficacy rather than optimal titration.
Conclusion
The current guidelines for PERT in EPI demonstrate substantial variability in dosing recommendations, both within and across disease types. This variation highlights the need for further research to optimize PERT dosing and improve patient outcomes. Healthcare providers should consider individualizing PERT dosing based on nutritional status and response to therapy, ensuring regular follow-up with patients for dose titrations with consideration that most guidelines are framed as initial doses rather than upper limits.



Similar content being viewed by others
Notes
Typically, PERT is dosed based on FIP or USP units, which are 1:1 with each other on lipase and 1:1 with PhEur units of lipase. FIP and PhEur are also 1:1 on amylase and protease. However, USP units differ for amylase and protease, where 1 PhEur or 1 FIP or 1 BP of amylase equals 4.15 USP units; and 1 PhEur of 1 FIP or 1 BP of protease equals 62.5 USP units. Source: Layer et al. 2001[114].
In this review, we refer to PhEur units specifically when they are used, because this will make a meaningful difference for future research studies that evaluates protein digestion using protease unit conversion. Regardless, readers can infer that all lipase units are 1:1 across all unit types within this paper and otherwise ignore unit types.
References
Diéguez-Castillo C, Jiménez-Luna C, Prados J, Martín-Ruiz JL, Caba O. State of the art in exocrine pancreatic insufficiency. Medicina (Buenos Aires) 2020;56:523. https://doi.org/10.3390/medicina56100523.
Singh VK, Haupt ME, Geller DE, Hall JA, Diez PMQ. Less common etiologies of exocrine pancreatic insufficiency. World J Gastroenterol. 2017. https://doi.org/10.3748/wjg.v23.i39.7059.
Capurso G, Traini M, Piciucchi M, Signoretti M, Arcidiacono PG. Exocrine pancreatic insufficiency: prevalence, diagnosis, and management. Clin Exp Gastroenterol. 2019. https://doi.org/10.2147/CEG.S168266.
Powell-Brett S, de Liguori Carino N, Roberts K. Understanding pancreatic exocrine insufficiency and replacement therapy in pancreatic cancer. Eur J Surg Oncol 2021. Published online. doi: https://doi.org/10.1016/j.ejso.2020.03.006.
Sikkens ECM, Cahen DL, van Eijck C, Kuipers EJ, Bruno MJ. Patients with exocrine insufficiency due to chronic pancreatitis are undertreated: a Dutch national survey. Pancreatology 2012;12:71–73. https://doi.org/10.1016/j.pan.2011.12.010.
Harzing AW. Publish or perish. https://www.harzing.com/resources/publish-or-perish.
Winstead NS, Wilcox CM. Clinical trials of pancreatic enzyme replacement for painful chronic pancreatitis—a review. Pancreatology. 2009. https://doi.org/10.1159/000212086.
Fragoso AV, Pedroso MR, Herman P, Montagnini AL. Comparing the enzyme replacement therapy cost in post pancreatectomy patients due to pancreatic tumor and chronic pancreatitis. Arq Gastroenterol. 2016. https://doi.org/10.1590/S0004-28032016000200008.
Konstan MW, Liou TG, Strausbaugh SD, et al. Efficacy and safety of a new formulation of pancrelipase (Ultrase MT20) in the treatment of malabsorption in exocrine pancreatic insufficiency in cystic fibrosis. Gastroenterol Res Pract 2010. Published online. doi: https://doi.org/10.1155/2010/898193.
Byelyayeva N, Gubergrits N, Lukashevich G. Exocrine pancreatic insufficiency in celiac disease: frequency and cause of low treatment efficacy. Pancreatology. 2020. https://doi.org/10.1016/j.pan.2020.07.204.
Domínguez-Muñoz JE, Nieto-Garcia L, López-Díaz J, Lariño-Noia J, Abdulkader I, Iglesias-Garcia J. Impact of the treatment of pancreatic exocrine insufficiency on survival of patients with unresectable pancreatic cancer: a retrospective analysis. BMC Cancer. 2018. https://doi.org/10.1186/s12885-018-4439-x.
Domínguez-Muñoz JE. Pancreatic enzyme replacement therapy: exocrine pancreatic insufficiency after gastrointestinal surgery. HPB. 2009. https://doi.org/10.1111/j.1477-2574.2009.00132.x.
Delhaye M, Van Steenbergen W, Cesmeli E et al. Belgian consensus on chronic pancreatitis in adults and children: statements on diagnosis and nutritional, medical, and surgical treatment. Acta Gastroenterol Belg 2014;77:47–65.
Turki S, Kallel H. Emerging approaches for the treatment of fat malabsorption due to exocrine pancreatic insufficiency. In: New Advances in the Basic and Clinical Gastroenterology. 2012. https://doi.org/10.5772/34278.
Kleeff J, Whitcomb DC, Shimosegawa T et al. Chronic pancreatitis. Nat Rev Dis Primers 2017;3:17060. https://doi.org/10.1038/nrdp.2017.60.
Littlewood JM, Wolfe SP, Conway SP. Diagnosis and treatment of intestinal malabsorption in cystic fibrosis. Pediatr Pulmonol. 2006. https://doi.org/10.1002/ppul.20286.
Pezzilli R, Caccialanza R, Capurso G, Brunetti O, Milella M, Falconi M. Pancreatic enzyme replacement therapy in pancreatic cancer. Cancers (Basel) 2020;12:275. https://doi.org/10.3390/cancers12020275.
Landers A, Brown H, Strother M. The effectiveness of pancreatic enzyme replacement therapy for malabsorption in advanced pancreatic cancer, a pilot study. Palliat Care Soc Pract. 2019. https://doi.org/10.1177/1178224218825270.
Roeyen G, Berrevoet F, Borbath I, et al. Expert opinion on management of pancreatic exocrine insufficiency in pancreatic cancer. ESMO Open 2022. Published online. doi: https://doi.org/10.1016/j.esmoop.2022.100386.
Phillips ME, Hopper AD, Leeds JS et al. Consensus for the management of pancreatic exocrine insufficiency: UK practical guidelines. BMJ Open Gastroenterol. 2021. https://doi.org/10.1136/bmjgast-2021-000643.
Brownell JN, Padula L, Reid E, Stallings VA, Maqbool A. Nutritional therapy, pancreatic exocrine insufficiency, and pancreatic enzyme replacement therapy in cystic fibrosis. In: Clinical Pancreatology for Practising Gastroenterologists and Surgeons. 2021. https://doi.org/10.1002/9781119570097.ch48.
Forsmark CE, Tang G, Xu H, Tuft M, Hughes SJ, Yadav D. The use of pancreatic enzyme replacement therapy in patients with a diagnosis of chronic pancreatitis and pancreatic cancer in the US is infrequent and inconsistent. Aliment Pharmacol Ther. 2020. https://doi.org/10.1111/apt.15698.
Kempeneers MA, Ahmed Ali U, Issa Y et al. Natural course and treatment of pancreatic exocrine insufficiency in a nationwide cohort of chronic pancreatitis. Pancreas. 2020. https://doi.org/10.1097/MPA.0000000000001473.
Sikkens EC, Cahen DL, van Eijck CH, Kuipers EJ, Bruno MJ. Practice of pancreatic enzyme replacement therapy in patients with exocrine insufficiency; a north European survey. Gastroenterology. 2011. https://doi.org/10.1016/s0016-5085(11)62266-x.
Subramaniam S, Nobar N, Besherdas K. Su1888 pancreatic enzyme replacement therapy in chronic pancreatitis and pancreatic cancer—are we getting it right? Gastroenterology. 2015. https://doi.org/10.1016/s0016-5085(15)31824-2.
Huddy JR, Macharg FMS, Lawn AM, Preston SR. Exocrine pancreatic insufficiency following esophagectomy. Dis Esophagus. 2013. https://doi.org/10.1111/dote.12004.
Moore JV, Tom S, Scoggins CR, Philips P, Egger ME, Martin RCG. Exocrine pancreatic insufficiency after pancreatectomy for malignancy: systematic review and optimal management recommendations. J Gastrointest Surg. 2021. https://doi.org/10.1007/s11605-020-04883-1.
Catarci M, Berlanda M, Grassi GB, Masedu F, Guadagni S. Pancreatic enzyme supplementation after gastrectomy for gastric cancer: a randomized controlled trial. Gastric Cancer. 2018. https://doi.org/10.1007/s10120-017-0757-y.
DiMagno EP. Controversies in the treatment of exocrine pancreatic insufficiency. Dig Dis Sci. 1982. https://doi.org/10.1007/BF01296724.
Barkin JA, Westermann A, Hoos W et al. Frequency of appropriate use of pancreatic enzyme replacement therapy and symptomatic response in pancreatic cancer patients. Pancreas. 2019. https://doi.org/10.1097/MPA.0000000000001330.
Mischler EH, Parrell S, Farrell PM, Odell GB. Comparison of effectiveness of pancreatic enzyme preparations in cystic fibrosis. Am J Dis Child. 1982. https://doi.org/10.1001/archpedi.1982.03970480026006.
Pap A. New emphasis in the treatment of pancreatic insufficiency. In: Cell Injury and Protection in the Gastrointestinal Tract. 1997. https://doi.org/10.1007/978-94-011-5392-8_31.
Brown A, Freedman SD. Pancreatic exocrine insufficiency. In: Diarrhea. Totowa: Humana Press; 2010:177–188. https://doi.org/10.1007/978-1-60761-183-7_10.
Anthony H, Catto-Smith A, Phelan P, Paxton S. Current approaches to the nutritional management of cystic fibrosis in Australia. J Paediatr Child Health 1998;34:170–174. https://doi.org/10.1046/j.1440-1754.1998.00193.x.
Lindkvist B. Diagnosis and treatment of pancreatic exocrine insufficiency. World J Gastroenterol 2013;19:7258. https://doi.org/10.3748/wjg.v19.i42.7258.
Roeyen G, Berrevoet F, Borbath I et al. Expert opinion on management of pancreatic exocrine insufficiency in pancreatic cancer. ESMO Open 2022;7:100386. https://doi.org/10.1016/j.esmoop.2022.100386.
Erchinger F, Tjora E, Nordaas IK et al. Pancreatic enzyme treatment in chronic pancreatitis: quality of management and adherence to guidelines—a cross-sectional observational study. United Eur Gastroenterol J 2022;10:844–853. https://doi.org/10.1002/ueg2.12276.
Struyvenberg MR, Martin CR, Freedman SD. Practical guide to exocrine pancreatic insufficiency—breaking the myths. BMC Med 2017;15:29. https://doi.org/10.1186/s12916-017-0783-y.
Lewis DM. An updated review of exocrine pancreatic insufficiency prevalence finds EPI is more common in general population than rates of co-conditions. J Gastrointest Liver Dis 2023; forthcoming.
Lewis DM. A systematic review of exocrine pancreatic insufficiency prevalence and treatment in type 1 and type 2 diabetes. Diabetes Technol Ther 2023;25:659–672. https://doi.org/10.1089/dia.2023.0157.
Pezzilli R, Andriulli A, Bassi C et al. Exocrine pancreatic insufficiency in adults: a shared position statement of the Italian Association for the Study of the Pancreas. World J Gastroenterol. 2013. https://doi.org/10.3748/wjg.v19.i44.7930.
Nofal YH, Abu Dail Y, Assaf Y, et al. Pancreatic enzyme replacement therapy for steatorrhoea in pancreatic cancer. Cochrane Database Syst Rev 2018. Published online February 12, 2018. doi: https://doi.org/10.1002/14651858.CD012952.
Sikkens ECM, Cahen DL, Kuipers EJ, Bruno MJ. Pancreatic enzyme replacement therapy in chronic pancreatitis. Best Pract Res Clin Gastroenterol 2010;24:337–347. https://doi.org/10.1016/j.bpg.2010.03.006.
Shandro BM, Nagarajah R, Poullis A. Challenges in the management of pancreatic exocrine insufficiency. World J Gastrointest Pharmacol Ther 2018. Published online. doi: https://doi.org/10.4292/wjgpt.v9.i5.39.
Muhammad WS, Alicia R, Melissa HS, Rachana P, Valerie R. Chronic use of long-acting somatostatin analogues (SSAs) and exocrine pancreatic insufficiency (EPI) in patients with gastroenteropancreatic neuroendocrine tumors (GEP-NETs): an under-recognized adverse effect. Cancer Med J 2020;3:75. https://doi.org/10.46619/Cmj.2020.3-1023.
Price DA, Schmid ML, Ong ELC, Adjukeiwicz KMB, Peaston B, Snow MH. Pancreatic exocrine insufficiency in HIV-positive patients. HIV Med. 2005. https://doi.org/10.1111/j.1468-1293.2005.00263.x.
Yilmaz A, Hagberg L. Exocrine pancreatic insufficiency is common in people living with HIV on effective antiretroviral therapy. Infect Dis. 2018. https://doi.org/10.1080/23744235.2017.1370126.
Graham DY. An enteric-coated pancreatic enzyme preparation that works. Dig Dis Sci. 1979. https://doi.org/10.1007/BF01311943.
Pezzilli R. Alcoholic chronic pancreatitis and exocrine pancreatic insufficiency. Adv Res Gastroenterol Hepatol. 2017. https://doi.org/10.19080/argh.2017.03.555618.
Kiefer T, Krahl D, Osthoff K et al. Importance of pancreatic enzyme replacement therapy after surgery of cancer of the esophagus or the esophagogastric junction. Nutr Cancer. 2018. https://doi.org/10.1080/01635581.2017.1374419.
Radlinger B, Ramoser G, Kaser S. Exocrine pancreatic insufficiency in type 1 and type 2 diabetes. Curr Diabetes Rep. 2020. https://doi.org/10.1007/s11892-020-01304-0.
Whitcomb DC, Bodhani A, Beckmann K et al. Efficacy and safety of pancrelipase/pancreatin in patients with exocrine pancreatic insufficiency and a medical history of diabetes mellitus. Pancreas. 2016. https://doi.org/10.1097/MPA.0000000000000514.
Mongil Poce L, Pérez Aísa Á, Alcaide J et al. Optimization of enzyme replacement treatment (ERT) for exocrine pancreatic insufficiency (EPI) in gastrectomy patients. Pancreatology. 2017. https://doi.org/10.1016/j.pan.2017.08.039.
Sridhar RP, Yacob M, Chowdhury SD, Balasubramanian KA, Samarasam I. Exocrine pancreatic insufficiency following gastric resectional surgery—is routine pancreatic enzyme replacement therapy necessary? Indian J Surg Oncol. 2021. https://doi.org/10.1007/s13193-021-01315-7.
Timmerhuis HC, Weiland CJS, Santvoort HC. Diagnosis and therapeutic approach to pancreatic exocrine insufficiency after acute pancreatitis. In: Clinical Pancreatology for Practising Gastroenterologists and Surgeons. 2021. https://doi.org/10.1002/9781119570097.ch21.
Srivoleti P, Yang AL, Jin DX, Banks PA, McNabb-Baltar J. Sa1390 management and surveillance of exocrine pancreatic insufficiency in patients with chronic pancreatitis. Gastroenterology. 2020. https://doi.org/10.1016/s0016-5085(20)31556-0.
de Rijk FEM, van Veldhuisen CL, Besselink MG et al. Diagnosis and treatment of exocrine pancreatic insufficiency in chronic pancreatitis: an international expert survey and case vignette study. Pancreatology 2022;22:457–465. https://doi.org/10.1016/j.pan.2022.03.013.
Somaraju UR, Solis-Moya A. Pancreatic enzyme replacement therapy for people with cystic fibrosis. In: Somaraju UR, ed. Cochrane Database of Systematic Reviews. Wiley; 2014. https://doi.org/10.1002/14651858.CD008227.pub2.
Somaraju UR, Solis-Moya A. Pancreatic enzyme replacement therapy for people with cystic fibrosis. Cochrane Database Syst Rev 2016. Published online November 23, 2016. doi: https://doi.org/10.1002/14651858.CD008227.pub3.
Somaraju URR, Solis-Moya A. Pancreatic enzyme replacement therapy for people with cystic fibrosis. Cochrane Database Syst Rev. 2020. https://doi.org/10.1002/14651858.CD008227.pub4.
Vecht J, Symersky T, Lamers CBHW, Masclee AAM. Efficacy of lower than standard doses of pancreatic enzyme supplementation therapy during acid inhibition in patients with pancreatic exocrine insufficiency. J Clin Gastroenterol 2006;40:721–725. https://doi.org/10.1097/00004836-200609000-00012.
Shandro B, Chen J, Ritehnia J, Poullis A. P259 abnormal pancreatic imaging and nutrition biochemistry predict response to pancreatic enzyme replacement therapy. In: Posters. BMJ Publishing Group Ltd and British Society of Gastroenterology; 2021:A175.2–A176. https://doi.org/10.1136/gutjnl-2020-bsgcampus.333.
Trestini I, Carbognin L, Peretti U et al. Pancreatic enzyme replacement therapy in patients undergoing first-line gemcitabine plus nab-paclitaxel for advanced pancreatic adenocarcinoma. Front Oncol. 2021. https://doi.org/10.3389/fonc.2021.688889.
Lewis DM, Shahid A. Survey of pancreatic enzyme replacement therapy dosing experiences in adults with exocrine pancreatic insufficiency. Healthcare 2023;11:2316.
Tang D, Sealock RJ. Diabetes and the exocrine pancreas. In: Managing Gastrointestinal Complications of Diabetes. 2017. https://doi.org/10.1007/978-3-319-48662-8_9.
Cui YF, Andersen DK. Pancreatogenic diabetes: special considerations for management. Pancreatology. 2011. https://doi.org/10.1159/000329188.
Cummings MH, Chong L, Hunter V, Kar PS, Meeking DR, Cranston ICP. Gastrointestinal symptoms and pancreatic exocrine insufficiency in type 1 and type 2 diabetes. Pract Diabetes. 2015. https://doi.org/10.1002/pdi.1924.
Foster TP, Bruggeman B, Campbell-Thompson M, Atkinson MA, Haller MJ, Schatz DA. Exocrine pancreas dysfunction in type 1 diabetes. Endocr Pract. 2020. https://doi.org/10.4158/EP-2020-0295.
Lewis DM, Shahid A. Glycemic variability assessment in newly treated exocrine pancreatic insufficiency with type 1 diabetes. J Diabetes Sci Technol 2022. Published online July 5, 2022. doi: https://doi.org/10.1177/19322968221108414.
Blonk L, Wierdsma NJ, Jansma EP, Kazemier G, Van Der Peet DL, Straatman J. Exocrine pancreatic insufficiency after esophagectomy: a systematic review of literature. Dis Esophagus. 2021. https://doi.org/10.1093/dote/doab003.
Blonk L, Wierdsma NJ, Jansma EP, Kazemier G, Peet DL, Straatman J. 727 Exocrine pancreatic insufficiency after esophagectomy: a systematic review of literature. Dis Esophagus. 2021. https://doi.org/10.1093/dote/doab052.727.
Shils ME. Nutritional problems associated with gastrointestinal and genitourinary cancer. Cancer Res 1977;37:2366–2372.
Friess H, Tempia-Caliera AA, Cammerer G, Büchler MW. Indication for pancreatic enzyme substitution following gastric resection. Pancreatology. 2001. https://doi.org/10.1159/000055891.
Straatman J, Wiegel J, Van Der Wielen N, Jansma EP, Cuesta MA, Van Der Peet DL. Systematic review of exocrine pancreatic insufficiency after gastrectomy for cancer. Dig Surg. 2017. https://doi.org/10.1159/000454958.
Antonini F, Crippa S, Falconi M, Macarri G, Pezzilli R. Pancreatic enzyme replacement therapy after gastric resection: an update. Dig Liver Dis. 2018. https://doi.org/10.1016/j.dld.2017.10.025.
Chaudhary A, Domínguez-Muñoz JE, Layer P, Lerch MM. Pancreatic exocrine insufficiency as a complication of gastrointestinal surgery and the impact of pancreatic enzyme replacement therapy. Dig Dis. 2020. https://doi.org/10.1159/000501675.
Huang W, de la Iglesia-García D, Baston-Rey I, et al. Exocrine pancreatic insufficiency following acute pancreatitis: systematic review and meta-analysis. Dig Dis Sci 2019. Published online. doi: https://doi.org/10.1007/s10620-019-05568-9.
Watson L. Exocrine insufficiency and pancreatic enzyme replacement therapy in pancreatic cancer. Clin Oncol 2010. Published online. doi: https://doi.org/10.1016/j.clon.2010.03.004.
Bartel MJ, Asbun H, Stauffer J, Raimondo M. Pancreatic exocrine insufficiency in pancreatic cancer: a review of the literature. Dig Liver Dis 2015. Published online. doi: https://doi.org/10.1016/j.dld.2015.06.015.
Saito T, Hirano K, Isayama H, et al. The role of pancreatic enzyme replacement therapy in unresectable pancreatic cancer: a prospective cohort study. Pancreas 2017. Published online. doi: https://doi.org/10.1097/MPA.0000000000000767.
Powell‐Brett S, Roberts KJ. Diagnosis and management of pancreatic exocrine insufficiency in pancreatic cancer. In: Clinical Pancreatology for Practising Gastroenterologists and Surgeons. 2021. https://doi.org/10.1002/9781119570097.ch66.
Woo SM, Joo J, Kim SY, et al. Efficacy of pancreatic exocrine replacement therapy for patients with unresectable pancreatic cancer in a randomized trial. Pancreatology 2016. Published online. doi: https://doi.org/10.1016/j.pan.2016.09.001.
Powell-Brett S, Chinuck R, Roberts K. Management of pancreatic exocrine insufficiency. In: Textbook of Pancreatic Cancer. 2021. https://doi.org/10.1007/978-3-030-53786-9_43.
Vujasinovic M, Valente R, Del Chiaro M, Permert J, Löhr JM. Pancreatic exocrine insufficiency in pancreatic cancer. Nutrients 2017. Published online. doi: https://doi.org/10.3390/nu9030183.
Rahib L, Westermann A, Barkin JA, et al. Frequency of appropriate use of pancreatic enzyme replacement therapy (PERT) and symptomatic response in pancreatic cancer patients. Am J Gastroenterol 2017. Published online. doi: https://doi.org/10.14309/00000434-201710001-00048.
Gilliland TM, Villafane-Ferriol N, Shah KP, et al. Nutritional and metabolic derangements in pancreatic cancer and pancreatic resection. Nutrients 2017. Published online. doi: https://doi.org/10.3390/nu9030243.
Barkin JA, Harb DM, Kort JM, Barkin JS. S44 analysis of real world patient experience with pancreatic enzyme replacement therapy (PERT) in the treatment of exocrine pancreatic insufficiency (EPI). Am J Gastroenterol. 2021. https://doi.org/10.14309/01.ajg.0000772156.58431.47.
Bruno MJ. Maldigestion and exocrine pancreatic insufficiency after pancreatic resection for malignant disease: pathophysiology and treatment. Pancreatology. 2001. https://doi.org/10.1159/000055893.
Kahl S, Malfertheiner P. Exocrine and endocrine pancreatic insufficiency after pancreatic surgery. Best Pract Res Clin Gastroenterol. 2004. https://doi.org/10.1016/S1521-6918(04)00089-7.
Pezzilli R. Diagnosis and therapy of exocrine pancreatic insufficiency after gastric and pancreatic surgery. In: Clinical Pancreatology for Practising Gastroenterologists and Surgeons. 2021. https://doi.org/10.1002/9781119570097.ch78.
Seiler CM, Izbicki J, Varga-Szabó L, Czakó L, Fiók J, Sperti C. A double-blind, randomized, placebo-controlled study of pancreatin 25,000 minimicrospheres for pancreatic exocrine insufficiency after major pancreatic resection. Pancreatology 2012;12:527. https://doi.org/10.1016/j.pan.2012.11.089.
Bodhani A, Whitcomb D, Beckmann K, Fuldeore M, Sander-Struckmeier S, Pollack P. Retrospective subgroup analysis to assess the efficacy and safety of pancrelipase/pancreatin (CREON®) in patients with exocrine pancreatic insufficiency (EPI) and a medical history of diabetes mellitus. Am J Gastroenterol. 2012. https://doi.org/10.14309/00000434-201210001-00194.
Seiler CM, Izbicki J, Varga-Szabõ L et al. Randomised clinical trial: a 1-week, double-blind, placebo-controlled study of pancreatin 25 000 Ph Eur minimicrospheres (Creon 25000 MMS) for pancreatic exocrine insufficiency after pancreatic surgery, with a 1-year open-label extension. Aliment Pharmacol Ther. 2013. https://doi.org/10.1111/apt.12236.
Phillips ME. Pancreatic exocrine insufficiency following pancreatic resection. Pancreatology. 2015. https://doi.org/10.1016/j.pan.2015.06.003.
Sabater L, Ausania F, Bakker OJ et al. Evidence-based guidelines for the management of exocrine pancreatic insufficiency after pancreatic surgery. Ann Surg. 2016. https://doi.org/10.1097/SLA.0000000000001732.
Pathanki AM, Attard JA, Bradley E et al. Pancreatic exocrine insufficiency after pancreaticoduodenectomy: current evidence and management. World J Gastrointest Pathophysiol. 2020. https://doi.org/10.4291/wjgp.v11.i2.20.
Petzel MQB, Hoffman L. Nutrition implications for long-term survivors of pancreatic cancer surgery. Nutr Clin Pract. 2017. https://doi.org/10.1177/0884533617722929.
Brownell JN, McKnight-Menci H, Maqbool A, Thornton PS. Management of diabetes and pancreatic insufficiency after pancreatectomy. In: Contemporary Endocrinology. 2019. https://doi.org/10.1007/978-3-030-02961-6_12.
Durie P. Uses and abuses of enzyme therapy in cystic fibrosis. J R Soc Med Suppl. 1998. https://doi.org/10.1177/014107689809134s02.
Anthony H, Collins CE, Davidson G et al. Pancreatic enzyme replacement therapy in cystic fibrosis: Australian guidelines. J Paediatr Child Health. 1999. https://doi.org/10.1046/j.1440-1754.1999.00363.x.
Pancreatic Enzymes Clinical Care Guidelines: Executive Summary. US Cystic Fibrosis Foundation; 2021. https://www.cff.org/medical-professionals/pancreatic-enzymes-clinical-care-guidelines. Accessed March 29, 2023.
Somayaji R, Ramos KJ, Kapnadak SG, Aitken ML, Goss CH. Common clinical features of CF (respiratory disease and exocrine pancreatic insufficiency). Presse Med. 2017. https://doi.org/10.1016/j.lpm.2017.03.021.
Stallings VA, Stark LJ, Robinson KA, Feranchak AP, Quinton H. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. J Am Diet Assoc. 2008. https://doi.org/10.1016/j.jada.2008.02.020.
Colombo C, Nobili RM, Alicandro G. Challenges with optimizing nutrition in cystic fibrosis. Expert Rev Respir Med. 2019. https://doi.org/10.1080/17476348.2019.1614917.
Taylor CJ, Thieroff-Ekerdt R, Shiff S, Magnus L, Fleming R, Gommoll C. Comparison of two pancreatic enzyme products for exocrine insufficiency in patients with cystic fibrosis. J Cyst Fibros. 2016. https://doi.org/10.1016/j.jcf.2016.02.010.
Trapnell BC, Maguiness K, Graff GR, Boyd D, Beckmann K, Caras S. Efficacy and safety of Creon® 24,000 in subjects with exocrine pancreatic insufficiency due to cystic fibrosis. J Cyst Fibros. 2009. https://doi.org/10.1016/j.jcf.2009.08.008.
Munck A. Nutritional considerations in patients with cystic fibrosis. Expert Rev Respir Med. 2010. https://doi.org/10.1586/ers.09.66.
Baker SS, Borowitz D, Baker RD. Pancreatic exocrine function in patients with cystic fibrosis. Curr Gastroenterol Rep. 2005. https://doi.org/10.1007/s11894-005-0039-4.
King CS, Brown AW, Aryal S, Ahmad K, Donaldson S. Critical care of the adult patient with cystic fibrosis. Chest. 2019. https://doi.org/10.1016/j.chest.2018.07.025.
Somaraju UR, Solis-Moya A. Pancreatic enzyme replacement therapy for people with cystic fibrosis. Cochrane Database Syst Rev. 2016. https://doi.org/10.1002/14651858.CD008227.pub3.
Graham DY. Treatment of steatorrhea in chronic pancreatitis. Hosp Pract. 1986. https://doi.org/10.1080/21548331.1986.11706556.
Lankisch PG. Enzyme treatment of exocrine pancreatic insufficiency in chronic pancreatitis. In: Digestion, vol. 54. 1993. https://doi.org/10.1159/000201099.
Layer P, Holtmann G. Pancreatic enzymes in chronic pancreatitis. Int J Pancreatol. 1994. https://doi.org/10.1007/BF02924382.
Layer P, Keller J, Lankisch PG. Pancreatic enzyme replacement therapy. Curr Gastroenterol Rep 2001;3:101–108. https://doi.org/10.1007/s11894-001-0005-8.
Lankisch PG, Lübbers H, Mahlke R. Medical management of chronic pancreatitis. In: Diseases of the Pancreas. Berlin: Springer:331–347. https://doi.org/10.1007/978-3-540-28656-1_37.
Löhr JM, Haas SL, Lindgren F et al. Conservative treatment of chronic pancreatitis. Dig Dis 2013;31:43–50. https://doi.org/10.1159/000345720.
Sirchak YS, Barani VY. Evaluation of substitution enzyme therapy of exocrine pancreatic insufficiency of pancreatic gland in patients with diabetes mellitus and chronic pancreatitis. Ukraïnsʹkij ž med bìol sportu 2020;5:163–169. https://doi.org/10.26693/jmbs05.06.163.
Iglesias-Garcia J, Iglesias-Garcia M, Iglesias-Rey M, Dominguez-Munoz E. Oral pancreatic enzyme supplementation in patients with exocrine pancreatic insufficiency: is it enough to evaluate clinical response? Gastroenterology. 2003. https://doi.org/10.1016/s0016-5085(03)83204-3.
Whitcomb D, Malecka-Panas E, Gubergrits N, Caras S, Shen Y, Sander-Struckmeier S. Effect of underlying disease type on efficacy and safety of pancrelipase delayed-release capsules (CREON®) in a randomized trial of patients with exocrine pancreatic insufficiency due to chronic pancreatitis or pancreatic surgery. Am J Gastroenterol 2010;105:S51–S52. https://doi.org/10.14309/00000434-201010001-00137.
Sikkens EC, Cahen DL, van Eijck CH, Kuipers EJ, Bruno MJ. M1368 prescription and response to pancreatic enzyme therapy in exocrine insufficiency due to chronic pancreatitis; a Dutch national survey. Gastroenterology 2010;138:S-390. https://doi.org/10.1016/S0016-5085(10)61792-1.
D’Haese JG, Ceyhan GO, Demir IE et al. Pancreatic enzyme replacement therapy in patients with exocrine pancreatic insufficiency due to chronic pancreatitis. Pancreas 2014;43:834–841. https://doi.org/10.1097/MPA.0000000000000131.
Fragoso AV, Pedroso MR, Herman P, Montagnini AL. Comparing the enzyme replacement therapy cost in post pancreatectomy patients due to pancreatic tumor and chronic pancreatitis. Arq Gastroenterol 2016;53:94–97. https://doi.org/10.1590/S0004-28032016000200008.
Kempeneers MA, Ahmed Ali U, Issa Y et al. Natural course and treatment of pancreatic exocrine insufficiency in a nationwide cohort of chronic pancreatitis. Pancreas 2020;49:242–248. https://doi.org/10.1097/MPA.0000000000001473.
Toskes PP, Secci A, Thieroff-Ekerdt R. M1388 a randomized, double-blind, dose-response control, crossover study of two doses of Eur-1008 (Zenpep) in chronic pancreatitis (CP) patients with exocrine pancreatic insufficiency (EPI). Gastroenterology 2010;138:S-394-S−395. https://doi.org/10.1016/S0016-5085(10)61812-4.
Toskes PP, Secci A, Thieroff-Ekerdt R. Efficacy of a novel pancreatic enzyme product, EUR-1008 (Zenpep), in patients with exocrine pancreatic insufficiency due to chronic pancreatitis. Pancreas 2011;40:376–382. https://doi.org/10.1097/MPA.0b013e31820b971c.
Morawski JH, Prüfert A, Van Engen A et al. Cost-effectiveness analysis of pancreatin minimicrospheres in patients with pancreatic exocrine insufficiency due to chronic pancreatitis. J Med Econ. 2012. https://doi.org/10.3111/13696998.2012.737882.
de-Madaria E, Abad-González A, Aparicio JR, et al. The Spanish Pancreatic Club’s recommendations for the diagnosis and treatment of chronic pancreatitis: Part 2 (treatment). Pancreatology 2013; 13: 18–28. doi: https://doi.org/10.1016/j.pan.2012.11.310.
Sheth SG, Conwell DL, Whitcomb DC et al. Academic Pancreas Centers of Excellence: guidance from a multidisciplinary chronic pancreatitis working group at PancreasFest. Pancreatology 2017;17:419–430. https://doi.org/10.1016/j.pan.2017.02.015.
O’Brien SJ, Omer E. Chronic pancreatitis and nutrition therapy. Nutr Clin Pract 2019;34:S13–S26. https://doi.org/10.1002/ncp.10379.
Singh VK, Yadav D, Garg PK. Diagnosis and management of chronic pancreatitis. JAMA 2019;322:2422. https://doi.org/10.1001/jama.2019.19411.
Löhr JM, Dominguez-Munoz E, Rosendahl J et al. United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU). United Eur Gastroenterol J 2017;5:153–199. https://doi.org/10.1177/2050640616684695.
Dominguez-Munoz JE, Drewes AM, Lindkvist B et al. Recommendations from the United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis. Pancreatology 2018;18:847–854. https://doi.org/10.1016/j.pan.2018.09.016.
Gardner TB, Adler DG, Forsmark CE, Sauer BG, Taylor JR, Whitcomb DC. ACG clinical guideline: chronic pancreatitis. Am J Gastroenterol 2020;115:322–339. https://doi.org/10.14309/ajg.0000000000000535.
Molero X, Ayuso JR, Balsells J et al. Pancreatitis crónica para el clínico. Parte 2: Tratamiento y seguimiento. Documento de posicionamiento interdisciplinar de la Societat Catalana de Digestologia y la Societat Catalana de Pàncrees. Gastroenterol Hepatol 2022;45:304–314. https://doi.org/10.1016/j.gastrohep.2021.05.016.
Ramesh H, Reddy N, Bhatia S et al. A 51-week, open-label clinical trial in India to assess the efficacy and safety of pancreatin 40000 enteric-coated minimicrospheres in patients with pancreatic exocrine insufficiency due to chronic pancreatitis. Pancreatology 2013;13:133–139. https://doi.org/10.1016/j.pan.2013.01.009.
DiMagno MJ, DiMagno EP. Chronic pancreatitis. Curr Opin Gastroenterol 2013;29:531–536. https://doi.org/10.1097/MOG.0b013e3283639370.
Erchinger F, Øvre AKN, Aarseth MM et al. Fecal fat and energy loss in pancreas exocrine insufficiency: the role of pancreas enzyme replacement therapy. Scand J Gastroenterol 2018;53:1132–1138. https://doi.org/10.1080/00365521.2018.1499801.
Brownell JN, Schall JI, Stallings VA. Pancreatic function in chronic pancreatitis. Pancreas 2019;48:1068–1078. https://doi.org/10.1097/MPA.0000000000001381.
De La Iglesia-García D, Huang W, Szatmary P et al. Efficacy of pancreatic enzyme replacement therapy in chronic pancreatitis: systematic review and meta-analysis. Gut. 2017. https://doi.org/10.1136/gutjnl-2016-312529.
Min M, Patel B, Han S et al. Exocrine pancreatic insufficiency and malnutrition in chronic pancreatitis: identification, treatment, and consequences. Pancreas. 2018. https://doi.org/10.1097/MPA.0000000000001137.
Pham A, Forsmark C. Chronic pancreatitis: review and update of etiology, risk factors, and management. F1000Res 2018;7:607. https://doi.org/10.12688/f1000research.12852.1.
Acknowledgments
Dana Lewis is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
None.
Author information
Authors and Affiliations
Contributions
Conceptualizations, DML; methodology, DML, CL, JB; validation, DML; formal analysis, DML; data curation, DML; coding of search results for inclusion, DML, JR, KA, AK, CL; writing-original draft preparation, DML; writing-review and editing, DML, JR, KA, AK, CL, JB; visualization, DML; project administration, DML. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no financial conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix: Overview of PERT Dosing Guidelines for EPI by/with Co-condition
Appendix: Overview of PERT Dosing Guidelines for EPI by/with Co-condition
Somatostatin Analogues (SSA)
EPI can be an underreported side effect of somatostatin analogs (SSAs). One 2020 study [45] evaluated 19 patients with evidence of steatorrhea and commenced PERT at 72,000 units of lipase/meal, with a 78% response rate to PERT. While no guidelines exist for EPI following SSA use, this study suggests 38% of people with SSA may end up with EPI, and it’s likely to be misdiagnosed, but that PERT can reduce the cost of using short-acting SA and antidiarrheals.
HIV
An early 2005 study on HIV is both the first study on PERT in those with EPI and HIV and also one of the first to show the individualized need for PERT [46]. Of 7 individuals taking a range of 10,000–60,000 units of lipase/meal, four needed 10,000 units of lipase/meal; two needed 20,000 units of lipase/meal; and one individual used 60,000 units of lipase/meal. More recently, a 2017 study evaluated 12 individuals (out of 29 with HIV) who started PERT and used 50,000 units of lipase/meal [47].
Alcohol-Related EPI
A 1979 study evaluated higher doses (10,800–43–200/meal) needed as compared to newer enteric coated enzymes with 6015 units of lipase/meal [48]. More recently, in 2017 it was suggested that 20,000 units of lipase for breakfast, 40,000 units of lipase for lunch and dinner, and 20,000 units of lipase/snack were needed; however, this is a mini-review piece that did not cite a source for these recommendations and there do not appear to be any studies that validate this recommendation as compared to those with other types of chronic pancreatitis and EPI [49].
Celiac Disease
A 2020 study on PERT in people with celiac disease found 21.4% cases of EPI (12/56 people with celiac), and when evaluated low-moderate doses of PERT ranging from 60,000 to 120,000 units of lipase day, that 16.1% of the time, EPI was the reason for apparent low treatment efficacy alongside celiac disease [10].
Diabetes
It was previously not widely known that PERT has been evaluated in people with diabetes [65]. A 2011 study on pancreatogenic diabetes found a need for 60,000 units of lipase/meal, in some cases supported with PPI, as validated by an MTG breath test [66]. A 2016 study on the safety and efficacy of PERT in diabetes randomized individuals to 72,000 units of lipase/meal and 36,000 units of lipase/snack [52]. A 2015 review stated that 40,000–50,000 units of lipase/meal and 10,000–25,000 units of lipase/snack were typical starting doses for people with diabetes and EPI [67]. A 2020 review agrees [51], specifying for both type 1 and type 2 diabetes, whereas a separate review in 2020 suggests a slightly lower starting range of 20,000–40,000 units/meal in type 1 diabetes and points out this and most dose guidance is derived from the cystic fibrosis and EPI guidelines, including those suggesting max 10,000 units of lipase/kg/day or 4000 units of lipase/g of fat ingested [68]. Although it does not include guidelines or PERT dosing information for the population of people with diabetes, an n = 1 pilot study evaluating glucose variability in someone with type 1 diabetes [69] shows how PERT can reduce glycemic variability and improve glycemic outcomes, even in those with ideal hemoglobin A1c (HbA1c) and time in target glucose range (mg/dL) metrics, illustrating how effective digestion of macronutrients (or lack of effective digestion, for those with undiagnosed or un- or under-treated EPI) plays a role in glucose outcomes.
Esophagectomy
Two studies have evaluated PERT in people following esophagectomy, with one studying 25,000 units of lipase/meal achieving 87% improvement in 2012 [26] and a second study in 2018 evaluating 25,000–50,000 units of lipase/meal [50]. In addition, there have been two review articles, one in which concluded PERT was beneficial following esophagectomy [70], whereas another says higher evidence studies are needed [71].
Gastric Surgery (and Gastric Cancer)
An early publication from 1977 indicates PERT for gastrointestinal and genitourinary cancers [72]. A 2001 review suggested that the optimal dosage of PERT does not follow strict rules but rather has to be adjusted individually, and did not provide starting guidelines [73]. In 2009, a study found that the recommended dosage ranged from 40,000 to 50,000 units of lipase/meal and 20,000 units of lipase/snack, resulting in a range of 160,000 units of lipase/day in three meals plus two snacks in the lower dose to up to 400,000 units of lipase/day in some people (80,000–100,000 units of lipase/meal and 40,000–50,000 units of lipase/snack) [12].
A 2017 study found that a subgroup of patients required higher enzyme doses, ranging from 175,000 units of lipase/day (based on 50,000 units of lipase/meal and 25,000 units of lipase/snack) up to half of patients requiring 240,000 units of lipase/day (80,000 units of lipase/meal) [53]. In contrast, another 2017 study recommended a mere 10,000 units of lipase for breakfast and 20,000 units of lipase for lunch and dinner [28]. Meanwhile, a systematic review from 2017 concluded that a minimum of 40,000–50,000 units of lipase/meal is necessary [74]. In 2018, a study recommended that PERT dosage should not be less than 40,000–50,000 units of lipase/meal and no more than 75,000 units of lipase/meal [75]. A 2019 review of Spanish (2016), Italian (2013), Romanian (2015), and Australasian (2015) guidelines found that the most recent guidelines suggest 75,000 units of lipase/meal and 50,000 units of lipase/snack, with dosage recommendations ranging between 25,000–75,000 units of lipase/meal and 10,000–50,000 units of lipase/snack [76]. Finally, a 2021 study found that a range of 30,000–40,000 units of lipase meal and 15,000–20,000 units of lipase snack was effective [54].
Acute Pancreatitis
There is less evidence specific to acute pancreatitis; a 2009 review points out there is not strong evidence for PERT with EPI from acute pancreatitis but says withholding PERT until further evidence is not justified [77]. Meanwhile, a 2021 review suggests 40,000–50,000 units of lipase/meal [55].
Pancreatic Cancer
In 2010, it was noted that there is a paucity of data for starting doses of PERT for EPI in pancreatic cancer patients and that it is needed in most cases [78]. A 2015 study suggested that 25,000–50,000 units of lipase are needed/meal [79], but most other studies in or after 2015 began to suggest or study at least 40,000–50,000 units of lipase/meal and 25,000 units of lipase/snack [17, 25, 80, 81]. A 2016 randomized controlled trial (RCT) suggested this would indicate a daily range of 150,000–225,000 units of lipase/day [82]. Two separate 2021 reviews agreed on 50,000–75,000 units of lipase/meal and 25,000–50,000 units of lipase/snack [4, 83], and another 2019 study similarly started at 50,000 units of lipase/meal and 25,000 units of lipase/snack, topped off with an additional 25,000 units of lipase based on 16–20 g additional fat [18].
Another batch of studies suggested even higher doses, as a 2017 review suggested 72,000–75,000 units of lipase/meal and 36,000–50,000 units of lipase for snacks [84]. A 2018 Cochrane review summarized the evidence for 72,000 meals and 36,000 snacks [42]. Another 2019 study looked at the frequency of prescription but not dosage and recommended 72,000/meal and 36,000/snack, citing studies ranging from 40,000 to 72,000/meal and 20,000–36,000/snack. It also pointed out that pancreatic cancer is not an “on label” indication for PERT [30].
A 2018 study on unresectable pancreatic cancer found that 200,000 to 400,000 units of lipase/day (median dose of 325,000 units of lipase day) effectively increased the survival rate by using PERT. In that study, the starting dose was 50,000 units of lipase/meal and 25,000 units of lipase/snack, and the median ended up at 75,000 units of lipase/meal and 50,000 units of lipase for snacks [11]. A 2020 study defined appropriate dosing as > 120,000 units of lipase/day, with only 5.5% of pancreatic cancer patients receiving adequate dosing [22].
In terms of real-world dose usage, 2021 study found that the median dose of PERT for patients with pancreatic cancer was 80,000 units of lipase/day and 42% of patients received doses of 70,000 units of lipase/day or less. The study agrees with previous recommendations clustering around 40,000–50,000 units of lipase/meal and 25,000 units of lipase/snack at starting dosage, and noted that patients receiving doses above 80,000 units of lipase/day did not report gastrointestinal symptoms [63]. Meanwhile, a 2017 study focused on the frequency of prescriptions but not dosage amounts [85]. In 2017, a study on nutritional needs in pancreatic cancer highlighted EPI but provided no guidelines, in contrast to other recommendations provided [86]. Another 2021 study reported that 36% of patients with pancreatic cancer received less than 40,000 units of lipase/meal, indicating that they were underdosed based on American College of Gastroenterology guidelines for EPI in chronic pancreatitis [87].
A 2022 review agreed with the previous recommendation cluster of 40,000–50,000 units of lipase/meal and 25,000 units of lipase/snack, stating that they were “in line with guidelines for chronic pancreatitis” as well. However, the review also pointed out a higher recommendation cluster of Spanish surgery guidelines, which suggest 72,000–75,000 units of lipase/meal and 36,000–50,000 units of lipase/snack, and a Spanish consensus on 75,000 units of lipase for unresectable pancreatic cancer [36].
All in all, dose guidance ranges widely for pancreatic cancer, from 25,000 units of lipase/meal to 72,000–75,000 units of lipase/meal as starting doses, and 20,000 up to 50,000 units of lipase as the starting dose for snacks. Studies have shown a range of total daily doses from below 70,000 up to 400,000 units of lipase/day, noting that the lower doses are not necessarily recommended and correlate with unresolved symptoms.
Pancreatic Surgery
Data on PERT for pancreatic surgery was generated in the late 1990s, and a 1999 review acknowledged that a large number of enzymes were needed, ranging from 72,000 to 432,000 units of lipase per day, with a significant pill burden for patients. A 2001 review suggested that it might look like 20,000 to 150,000 units of lipase/meal, emphasizing that fewer pills were required with an enteric coating [88]. In 2004, the recommended dosage was between 40,000 and 120,000 units of lipase/meal [89], and ‘at least’ 40,000–50,000 units of lipase/meal has persisted as general guidance as recently as 2021 [90].
More recent studies have provided additional recommendations for higher doses, even with modern-era PERT. A 2012 RCT suggested a daily dose of 225,000 to 375,000 units of lipase, with 75,000 units of lipase/meal and 25,000 units of lipase/snack [91]. Another 2012 study recommended starting at 50,000 to 75,000 units of lipase/meal and 25,000 units of lipase/snack, with individual titration of up to 400,000 units of lipase/day as needed [92]. A 2013 RCT found that 75,000 PhEur units of lipase/meal and 50,000 units of lipase/snack were an appropriate, albeit high, starting dose for non-CF patients with exocrine pancreatic insufficiency (EPI) [93] pointing again to how many of the co-conditions are often anchored on or compared to CF-related guidelines for EPI. Subsequent reviews including one as recent as 2020 have supported these findings [94,95,96]. A 2017 review also stated that the varying range of dosages often reflects the fact that some patients self-restrict meal sizes and fat content and therefore require lower amounts of PERT, while others consume normal-sized meals with large amounts of fat, necessitating larger amounts of PERT [97].
In contrast, another 2017 review suggested dosing based on weight [98]. Surprisingly, a 2021 review followed, suggesting starting with 36,000 units of lipase or up to 72,000 units of lipase/meal, suggesting dosing at 36,000 units of lipase for those under 160 pounds and 72,000 units of lipase for those over 160 pounds. However, there is no evidence for a weight-based recommendation [27].
Like pancreatic cancer, dose guidance ranges widely for PERT in EPI and pancreatic surgery, typically from 50,000 up to 72,000–75,000 units of lipase/meal as starting doses; 25,000 up to 50,000 units of lipase as the starting dose for snacks, and up to 400,000 units of lipase/day or no more than 10,000 units of lipase/kg/day.
Cystic Fibrosis
Perhaps uniquely, many PERT dosing guidelines seem to emerge following the guidelines for CF and EPI. Much of the early studies on PERT are in CF. The CF community has the clearest and most sustained set of guidelines for EPI with less variance than other co-conditions.
For example, US guidelines emerged around 1995. Subsequent studies and guidelines cite or compare to those, such as a 1998 study in Australia evaluating real-world practice of clinicians who sometimes recommend patients receive 2,500 units of lipase/kg of body weight for meals in contrast to the US guidelines that suggest 500 units of lipase/kg of body weight for meals and 250 units of lipase/kg of body weight [99]. This highlights some of the variances in PERT guidelines globally and how they have changed slightly over time. In 1998 the US Cystic Fibrosis Foundation updated its guidelines [100] to recommend a maximum of 4000 units of lipase/g of fat/meal, up to a maximum of 10,000 units of lipase/kg of body weight/day (which would be 2500 units of lipase/kg of body weight up to 4 meals a day); however these guidelines remain in place and were evaluated in 2021 to still be sufficient as-is [101].
Many would recognize these numbers as cited throughout many EPI PERT dosing recommendations regardless of condition, from 2008 to 2021 [21, 102,103,104,105], and now are also used by other co-conditions, especially when there are no specific guidelines.
Some studies of PERT in CF such as one in 2009 observed that mean doses sometimes occur above the recommended 4000 units of lipase/g of fat level as well as above the total daily dose recommendation of 10,000 units of lipase/kg of body weight, which suggests this is not a hard limit [106]. The guidelines generated in 1998 may have still been influenced by early-era PERT, which was not regulatory approved and sometimes had over-fill issues. Similarly, a 2010 study observed a mean daily lipase dose of 4472 units of lipase/gram of fat and 16,941 units of lipase/kg of body weight as the total daily dose, which is much higher than the recommended 10,000 units of lipase/kg amount [9]. In 2010, a review suggested 250,000 units of lipase max, rather than according to body weight, for a total daily dose limit [107]. Similarly, a suggestion based on an overall amount of lipase per meal was first introduced in 2005 [108], whereas a more recent 2019 review suggests a starting dose of 40,000–50,000 units of lipase/meal [109].
It is also worth noting that, among the evidence in people with CF in EPI, Cochrane reviews from 2014 [58], 2016 [110], and 2020 [60] found no evidence on the relative dosages of enzymes needed for people with different levels of severity of pancreatic insufficiency.
In general, the CF guidelines from the US around PERT usage in EPI have persisted for nearly two decades and across other co-conditions with EPI. However, the evidence reviewed suggests that the ‘caps’ recommended may need to be updated or clarified, as there is little physiological evidence in post-2000 era PERT studies suggesting issues with larger PERT dosing than 10,000 units of lipase/kg of body weight or greater than 4000 units of lipase/g of fat consumed, especially as dietary fat consumption may vary widely from individual to individual.
Chronic Pancreatitis
In 1987, it was noted that unencapsulated enzyme doses of 10,000–12,000 units of lipase meal led to a 50% reduction in steatorrhea, and a quadrupling (e.g. 40,000–48,000 units of lipase), but not doubling, of the enzyme dose resulted in additional steatorrhea reduction [111]. A 1993 review suggested that doses should range between 20,000 and 40,000 units of lipase/meal or 100,000 units of lipase/day, with doses exceeding 250,000 units of lipase/day not being uncommon. It emphasized the importance of individualized dosing [112]. A 1994 review recommended a starting dose of 25,000–40,000 units of lipase meal, followed by a doubling of the dose, then reducing fat intake (50–75 g fat) to assess the effects, and finally adding a proton pump inhibitor (PPI) [113], supported by reviews and studies between 2001 and 2013 [114,115,116].
A 2020 review suggested a dose of 1000 units/kg of body weight or 25,000–50,000 units of lipase/meal [117]. Studies from 2003 [118] and 2009 [7] showed a broad range of doses, suggesting that doses could be more effectively optimized based on clinical observation. A 2006 study found no difference in enzyme dose by disease type in chronic pancreatitis (CP) and pancreatic surgery, with 72,000 units of lipase/meal and 36,000 units of lipase/snack being used [61]. Another 2010 study on the efficacy and safety of an enzyme product also found no difference by disease type [119].
In a 2010 study, the median starting dose at diagnosis was 100,000 units of lipase/day, with 63% of patients experiencing steatorrhea-like symptoms. After a median duration of 75 months, the dose increased to a median of 150,000 units of lipase/day, but 69% of patients still reported symptoms, suggesting undertreatment still occurred [120]. Other studies from 2014 [121], 2016 [122], and 2020 [123] support that undertreatment often occurs in EPI and CP. A 2022 study found that 2–39% of patients were not treated with pancreatic enzyme replacement therapy (PERT) when indicated [37].
A 2010 study found no difference between a 'high' dose of 140,000 units of lipase/day and a ‘low’ dose of 35,000 units of lipase/day, but the study had limitations, such as a short duration and not reporting meal content or quantity [124]. Another study in 2011 found no difference between high and low doses, but the high dose was effective in people with severe EPI [125]. A 2010 review suggested that dosing recommendations now range from 25 to 75,000 units of lipase/meal and 10,000 to 25,000 units of lipase/snack, with the majority of patients being adequately treated between 150 and 300,000 units of lipase/day. However, it also recommended starting with 50–75,000 units of lipase/meal and 25,000 units of lipase/snack or dosing by fat intake up to 400,000 units of lipase/day [43]. A 2012 study on cost-effectiveness used 40,000 units of lipase/meal and 25,000 units of lipase/snack based on Polish guidelines, while others recommended 40–50,000 units of lipase/meal and 10–25,000 units of lipase/snack [126].
In 2013, Spanish guidelines for treating CP suggested a minimum of 40–50,000 units of lipase/meal and 20,000–25,000 units of lipase/snack [127], and a 2014 Belgian consensus on CP supported these guidelines [13]. A 2017 guideline referred to cystic fibrosis (CF) guidelines for weight-based dosing (e.g., 500 units of lipase/kg to 2500 units of lipase/kg) [128], and one 2019 review also supported this approach [129] while another 2019 review suggested a dose of 1000 units of lipase/kg of body weight [130]. However, the 2017 HanPanEU guidelines [131], the 2018 United European Gastroenterology (UEG) guidelines [132], the 2020 American College of Gastroenterology (ACG) guidelines [133], and another 2022 review [134] all concur with the 40,000–50,000 units of lipase/meal and 25,000 units of lipase/snack recommendations.
Some higher doses have also been evaluated in EPI with CP. A 2013 study used an 80,000 units of lipase/meal and 40,000 units/snack dosing regimen [135], while another 2013 review pointed to 90,000 units of lipase/meal dosing [136]. A 2018 study found that 75,000 units of lipase/meal led to significant reductions in fecal fat and energy loss [137], and a 2019 study used 72,000 units of lipase/meal and 36,000 units of lipase/snack [138]. A 2017 meta-analysis concluded that doses between 100,000 units of lipase/meal and 400,000 units of lipase/meal might be needed in EPI and CP [139]. A 2018 study found that the median dose was 96,000 units of lipase/meal [140]. Another 2018 study agreed with the latest guidelines, suggesting that doses of up to 90,000 units of lipase/meal might be necessary [141].
In summary, the guidelines and dosing studies on CP and EPI are the most extensive, yet vary considerably more widely than the CF guidelines, and heavily cluster around 40,000–50,000 units of lipase/meal and 25,000–36,000,000 units of lipase/snack. However, real-world data and studies have evaluated higher doses between 72,000 and 96,000 units of lipase/meal, suggesting it is not uncommon to need to titrate higher than the typical starting doses recommended.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lewis, D.M., Rieke, J.G., Almusaylim, K. et al. Exocrine Pancreatic Insufficiency Dosing Guidelines for Pancreatic Enzyme Replacement Therapy Vary Widely Across Disease Types. Dig Dis Sci 69, 615–633 (2024). https://doi.org/10.1007/s10620-023-08184-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-023-08184-w