Abstract
Background
Sodium butyrate (NaB) is a short-chain fatty acid produced by intestinal microbial fermentation of dietary fiber, and has been shown to be effective in inhibiting ulcerative colitis (UC). However, how NaB regulates inflammation and oxidative stress in the pathogenesis of UC is not clear.
Aims
The purpose of this study was to use a dextran sulfate sodium salt (DSS)-induced murine colitis model, and determine the effects of NaB and the related molecular mechanisms.
Methods
Colitis model was induced in mice by administration of 2.5%(wt/vol) DSS. 0.1 M NaB in drinking water, or intraperitoneal injection of NaB (1 g/kg body weight) was given during the study period. In vivo imaging was performed to detect abdominal reactive oxygen species (ROS). Western blotting and RT-PCR were used to determine the levels of target signals.
Results
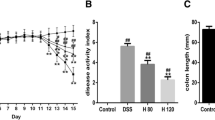
The results showed that NaB decreases the severity of colitis as determined by an improved survival rate, colon length, spleen weight, disease activity index (DAI), and histopathological changes. NaB reduced oxidative stress as determined by a reduction in abdominal ROS chemiluminescence signaling, inhibition of the accumulation of myeloperoxidase and malondialdehyde, and restoration of glutathione activity. NaB activated the COX-2/Nrf2/HO-1 pathway by increasing the expressions of COX-2, Nrf2, and HO-1 proteins. NaB inhibited the phosphorylation of NF-κB and activation of NLRP3 inflammasomes, and reduced the secretion of corresponding inflammatory factors. Furthermore, NaB promoted the occurrence of mitophagy via activating the expression of Pink1/Parkin.
Conclusions
In conclusion, our results indicate that NaB improves colitis by inhibiting oxidative stress and NF-κB/NLRP3 activation, which may be via COX-2/Nrf2/HO-1 activation and mitophagy.
Graphical Abstract







Similar content being viewed by others
Abbreviations
- NaB:
-
Sodium butyrate
- DSS:
-
Sulfate sodium salt
- UC:
-
Ulcerative colitis
- IBD:
-
Inflammatory bowel disease
- CRC:
-
Colorectal cancer
- SCFAs:
-
Short-chain fatty acids
- p.o.:
-
Orally
- i.p.:
-
Intraperitoneally
- DAI:
-
Disease activity index
- ROS:
-
Reactive oxygen species
- mtROS:
-
Mitochondrial reactive oxygen species
- mtDNA:
-
Mitochondrial DNA
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- Nrf2:
-
Nuclear factor erythrocyte 2-associated factor 2
- COX-2:
-
Cyclooxygenase-2
- EFOX:
-
Electrophilic oxo-derivatives
- HO-1:
-
Heme oxygenase-1
- MPO:
-
Myeloperoxidase
- MDA:
-
Malondialdehyde
- GSH:
-
Glutathione
- ASC:
-
Apoptosis-associated speck-like protein containing CARD
References
Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel J. Ulcerative colitis. Lancet (London, England) 2017;389:1756–1770.
Feuerstein JD, Moss AC, Farraye FA. Ulcerative colitis. Mayo Clin Proc 2019;94:1357–1373.
Nadeem MS, Kumar V, Al-Abbasi FA, Kamal MA, Anwar F. Risk of colorectal cancer in inflammatory bowel diseases. Semin Cancer Biol 2020;64:51–60.
D.E. O'Sullivan, R.L. Sutherland, S. Town, et al., Risk factors for early-onset colorectal cancer: a systematic review and meta-analysis. Clinical gastroenterology and hepatology 2021.
Kaplan GG. The global burden of IBD: from 2015 to 2025. Nature reviews. Gastroenterology & hepatology 2015;12:720–727.
Cottone M, Renna S, Modesto I, Orlando A. Is 5-ASA still the treatment of choice for ulcerative colitis? Curr Drug Targets 2011;12:1396–1405.
Baker DE, Kane S. The short- and long-term safety of 5-aminosalicylate products in the treatment of ulcerative colitis. Reviews in gastroenterological disorders 2004;4:86–91.
Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y, Dong W. ROS and ROS-mediated cellular signaling. Oxid Med Cell Longev 2016;2016:4350965.
Wang Z, Li S, Cao Y, Tian X, Zeng R, Liao D, Cao D. Oxidative stress and carbonyl lesions in ulcerative colitis and associated colorectal cancer. Oxid Med Cell Longev 2016;2016:9875298.
Aviello G, Knaus UG. ROS in gastrointestinal inflammation: rescue or sabotage? Brit J Pharmacol 2017;174:1704–1718.
Masi A, Fortini P, Krokidis MG et al. Increased levels of 5’,8-Cyclopurine DNA lesions in inflammatory bowel diseases. Redox Biol 2020;34:101562.
Morgan MJ, Liu Z. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res 2011;21:103–115.
Groeger AL, Cipollina C, Cole MP et al. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nat Chem Biol 2010;6:433–441.
Chen C. COX-2’s new role in inflammation. Nat Chem Biol 2010;6:401–402.
Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cellular and molecular life sciences: CMLS 2016;73:3221–3247.
Khor TO, Huang M, Kwon KH, Chan JY, Reddy BS, Kong A. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res 2006;66:11580–11584.
Y. Chen, P. Zhang, W. Chen, G. Chen, Ferroptosis mediated DSS-induced ulcerative colitis associated with Nrf2/HO-1 signaling pathway. Immunol Lett, 2020, 225.
Li J, Wang H, Zheng Z et al. Mkp-1 cross-talks with Nrf2/Ho-1 pathway protecting against intestinal inflammation. Free radical biology & medicine 2018;124:541–549.
Wang R, Luo Y, Lu Y, Wang D, Wang T, Pu W, Wang Y. Maggot extracts alleviate inflammation and oxidative stress in acute experimental colitis via the activation of Nrf2. Oxid Med Cell Longev 2019;2019:4703253.
Mei Y, Wang Z, Zhang Y et al. FA-97, a new synthetic caffeic acid phenethyl ester derivative, ameliorates DSS-induced colitis against oxidative stress by activating Nrf2/HO-1 pathway. Front Immunol 2019;10:2969.
Próchnicki T, Latz E. Inflammasomes on the crossroads of innate immune recognition and metabolic control. Cell Metab 2017;26:71–93.
Bauer C, Duewell P, Mayer C et al. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 2010;59:1192–1199.
Huber S, Gagliani N, Zenewicz LA et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 2012;491:259–263.
Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti T. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 2010;32:379–391.
Zhen Y, Zhang H. NLRP3 Inflammasome and inflammatory bowel disease. Front Immunol 2019;10:276.
M. Cornut, E. Bourdonnay, T. Henry, Transcriptional regulation of inflammasomes. Int J Mol Sci, 2020, 21.
Shimada K, Crother TR, Karlin J et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 2012;36:401–414.
Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011;469:221–225.
Priault M, Salin B, Schaeffer J, Vallette FM, di Rago J, Martinou J. Impairing the bioenergetic status and the biogenesis of mitochondria triggers mitophagy in yeast. Cell Death Differ 2005;12:1613–1621.
Matsuda N, Sato S, Shiba K et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. The Journal of cell biology 2010;189:211–221.
Y. Xu, J. Shen, Z. Ran, Emerging views of mitophagy in immunity and autoimmune diseases. Autophagy, 2020, 16.
Xiao L, Xu X, Zhang F et al. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol 2017;11:297–311.
Ryoo I, Kwak M. Regulatory crosstalk between the oxidative stress-related transcription factor Nfe2l2/Nrf2 and mitochondria. Toxicol Appl Pharm 2018;359:24–33.
Dinkova-Kostova AT, Abramov AY. The emerging role of Nrf2 in mitochondrial function. Free radical biology & medicine 2015;88:179–188.
Guo W, Sun Y, Liu W et al. Small molecule-driven mitophagy-mediated NLRP3 inflammasome inhibition is responsible for the prevention of colitis-associated cancer. Autophagy 2014;10:972–985.
Liu C, Wang J, Yang Y et al. Ginsenoside Rd ameliorates colitis by inducing p62-driven mitophagy-mediated NLRP3 inflammasome inactivation in mice. Biochem Pharmacol 2018;155:366–379.
Deleu S, Machiels K, Raes J, Verbeke K, Vermeire S. Short chain fatty acids and its producing organisms: an overlooked therapy for IBD? Ebiomedicine 2021;66:103293.
D. Parada Venegas, M.K. De la Fuente, G. Landskron, et al., Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol, 2019, 10, 277.
X. Dou, N. Gao, D. Yan, A. Shan, Sodium butyrate alleviates mouse colitis by regulating gut microbiota dysbiosis. Animals, 2020, 10.
Park J, Kotani T, Konno T et al. Promotion of intestinal epithelial cell turnover by commensal bacteria: role of short-chain fatty acids. Plos One 2016, 11, e156334
Silva JPB, Navegantes-Lima KC, Oliveira ALB et al. Protective mechanisms of butyrate on inflammatory bowel diseas. Curr Pharm Design 2018;24:4154–4166.
Luzardo-Ocampo I, Loarca-Piña G, Gonzalez De Mejia E. Gallic and butyric acids modulated NLRP3 inflammasome markers in a co-culture model of intestinal inflammation. Food and chemical toxicology 2020;146:111835.
Singh V, Yeoh BS, Walker RE et al. Microbiota fermentation-NLRP3 axis shapes the impact of dietary fibres on intestinal inflammation. Gut 2019;68:1801–1812.
Berndt BE, Zhang M, Owyang SY et al. Butyrate increases IL-23 production by stimulated dendritic cells. American journal of physiology. Gastrointestinal and liver physiology 2012;303:G1384–G1392.
Vieira ELM, Leonel AJ, Sad AP et al. Oral administration of sodium butyrate attenuates inflammation and mucosal lesion in experimental acute ulcerative colitis. The Journal of nutritional biochemistry 2012;23:430–436.
J.J. Kim, M.S. Shajib, M.M. Manocha, W.I. Khan, Investigating intestinal inflammation in DSS-induced model of IBD. Journal of visualized experiments : JoVE, 2012.
Liu Y, Tang B, Wang F et al. Parthenolide ameliorates colon inflammation through regulating Treg/Th17 balance in a gut microbiota-dependent manner. Theranostics 2020;10:5225–5241.
Xu M, Tao J, Yang Y et al. Ferroptosis involves in intestinal epithelial cell death in ulcerative colitis. Cell Death Dis 2020;11:86.
Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011;140:1785–1794.
Wirtz S, Popp V, Kindermann M, Gerlach K, Weigmann B, Fichtner-Feigl S, Neurath MF. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat Protoc 2017;12:1295–1309.
Donovan JD, Bauer L, Fahey GC, Lee Y. In vitro digestion and fermentation of microencapsulated tributyrin for the delivery of butyrate. J Food Sci 2017;82:1491–1499.
Asghar MN, Emani R, Alam C et al. In vivo imaging of reactive oxygen and nitrogen species in murine colitis. Inflamm Bowel Dis 2014;20:1435–1447.
Atreya I, Atreya R, Neurath MF. NF-kappaB in inflammatory bowel disease. J Intern Med 2008;263:591–596.
Jiang L, Wang J, Liu Z, Jiang A et al. Sodium butyrate alleviates lipopolysaccharide-induced inflammatory responses by down-regulation of NF-κB, NLRP3 signaling pathway, and activating histone acetylation in bovine macrophages. Front Vet Sci 2020;5(7):579674.
Li X, Wang C, Zhu J et al. Sodium butyrate ameliorates oxidative stress-induced intestinal epithelium barrier injury and mitochondrial damage through AMPK-mitophagy pathway. Oxid Med Cell Longev 2022;2022(29):3745135.
Feng Y, Wang Y, Wang P, Huang Y, Wang F. Short-chain fatty acids manifest stimulative and protective effects on intestinal barrier function through the inhibition of NLRP3 inflammasome and autophagy. Cellular Physiology and Biochemistry: International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology 2018;49:190–205.
Ahmed SMU, Luo L, Namani A, Wang XJ, Tang X. Nrf2 signaling pathway: pivotal roles in inflammation. Biochimica et biophysica acta. Molecular basis of disease 2017;1863:585–597.
Biasizzo M, Kopitar-Jerala N. Interplay between NLRP3 inflammasome and autophagy. Front Immunol 2020;11:591803.
Bauer C, Duewell P, Mayer C et al. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 2010;1192–1199.
Funding
This study was supported by National Natural Science Foundation of China (No. 81773429) and Natural Science Foundation of Guangdong Province, China (No. 2022A1515011631).
Author information
Authors and Affiliations
Contributions
Study idea, design and manuscript preparation: S.S., Z.B., and Q.Z. Data collection and interpretation: Z.B., Y.Q., and Y.Y. Experiment performance and data analysis: Z.B., X.S., L.L., H.L., and L.M. Final correction and review: W.L, L.Z., and S.S.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Animal welfare statement
All animal experiments followed the Guidelines for the Care and Use of Laboratory Animals, and the study was approved by Ethics Committee of Southern Medical University and Guangdong Medical Laboratory Animal Center (C202011-1). The animal studies are done according to ethical procedures and experimental protocols were approved in accordance with the Southern Medical University Experimental Animal Ethics Committee (L2019074). All experiments also complied with the Chinese regulations regarding animal experimentation.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bian, Z., Zhang, Q., Qin, Y. et al. Sodium Butyrate Inhibits Oxidative Stress and NF-κB/NLRP3 Activation in Dextran Sulfate Sodium Salt-Induced Colitis in Mice with Involvement of the Nrf2 Signaling Pathway and Mitophagy. Dig Dis Sci 68, 2981–2996 (2023). https://doi.org/10.1007/s10620-023-07845-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-023-07845-0