Abstract
Background
The novel peptide neuropeptide W (NPW) was originally shown to function in the control of feeding behavior and energy homeostasis. The aim of this study was to elucidate the putative preventive and therapeutic effects of NPW on colitis-associated oxidative injury and the underlying mechanisms for its action.
Methods
Sprague–Dawley rats in the acute colitis groups received NPW (0.5, 1 or 5 µg/kg/day) injections prior to induction of colitis with acetic acid, while the chronic colitis groups were treated after the induction of colitis. In both acute and chronic colitis (CC) groups, treatments were continued for 5 days and the rats were decapitated at the 24th hour of the last injections and colon tissues were collected for assessments.
Results
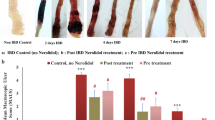
NPW pretreatment given for 5 days before colitis induction, as well as treating rats with NPW during the 5-day course of CC, abolished colonic lipid peroxidation. NPW treatment prevented colitis-induced reduction in blood flow, diminished neutrophil infiltration, and pro-inflammatory cytokine responses. NPW pretreatment only at the higher dose reduced colonic edema and microscopic score and preserved colonic glutathione stores. Elevations in cyclooxygenase (COX) enzyme activity and COX-1 protein level during the acute phase of colitis as well as reduction in COX-2 were all reversed with NPW pretreatment. In contrast, NPW treatment was effective in reducing the elevated COX-2 concentration during the chronic phase.
Conclusions
NPW alleviates acetic acid-induced oxidative colonic injury in rats through the upregulation of colonic blood flow as well as the inhibition of COX-2 protein expression and pro-inflammatory cytokine production.





Similar content being viewed by others
References
Feldman M, Friedman LS, Brandt LJ, Sleisenger and Fordtran's gastrointestinal and liver disease: pathophysiology, diagnosis, management, Elsevier Health Sciences 2020.
Blonski W, Buchner AM, Lichtenstein GR. Treatment of ulcerative colitis. Curr Opin Gastroenterol 2014;30:84–96.
Jairath V, Feagan BG. Global burden of inflammatory bowel disease. Lancet Gastroenterol Hepatol 2020;5:2–3.
Kumar S, Kumar A, Microbial pathogenesis in inflammatory bowel diseases. Microb Pathog 2021;105383.
Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med 2011;365:1713–1725.
Ghatule R, Shalini G, Gautam M, Singh A, Joshi V, Goel R. Effect of Azadirachta indica leaves extract on acetic acid-induced colitis in rats: Role of antioxidants, free radicals and myeloperoxidase. Asian Pac J Trop Dis 2012;2:S651–S657.
Hadji H, Bouchemal K, Advances in the treatment of inflammatory bowel disease: Focus on polysaccharide nanoparticulate drug delivery systems. Adv Drug Deliv Rev 2022;114101.
Jena G, Trivedi PP, Sandala B. Oxidative stress in ulcerative colitis: an old concept but a new concern. Free Radic Res 2012;46:1339–1345.
Singer II, Kawka DW, Schloemann S, Tessner T, Riehl T, Stenson WF. Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology 1998;115:297–306.
Tian T, Wang Z, Zhang J, Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxid Med Cell Longev 2017;2017.
Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology 1995;109:1344–1367.
Fabia R, Willén R, Ar’Rajab A, Andersson R, Ahrén B, Bengmark S. Acetic acid-induced colitis in the rat: a reproducible experimental model for acute ulcerative colitis. Eur Surg Res 1992;24:211–225.
Kornbluth A, Sachar D, Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, practice parameters committee. Am J Gastroenterol 2010;105: 501–23.
Baumgart DC. The diagnosis and treatment of Crohn’s disease and ulcerative colitis. Dtsch Ärztebl Int 2009;106:123.
Shimomura Y, Harada M, Goto M et al. Identification of neuropeptide W as the endogenous ligand for orphan G-protein-coupled receptors GPR7 and GPR8. J Biol Chem 2002;277:35826–35832.
Takenoya F, Kageyama H, Hirako S et al. Neuropeptide W. Front Endocrinol 2012;3:171.
Chottova Dvorakova M. Distribution and function of neuropeptides W/B signaling system. Front Physiol 2018;9:981.
Mondal MS, Yamaguchi H, Date Y et al. A role for neuropeptide W in the regulation of feeding behavior. Endocrinology 2003;144:4729–4733.
Yan F, Wang R, Li S et al. FoxO3a suppresses neuropeptide W expression in neuronal cells and in rat hypothalamus and its implication in hypothalamic–pituitary–adrenal (HPA) axis. Int J Biol Sci 2020;16:2775.
Ji L, Zhu H, Chen H et al. Modulation of CaV1.2 calcium channel by neuropeptide W regulates vascular myogenic tone via G protein-coupled receptor 7. J Hypertens 2015;33:2431–2442.
Pate AT, Yosten GL, Samson WK. Neuropeptide W increases mean arterial pressure as a result of behavioral arousal. Am J Physiol Regul Integr Comp Physiol 2013;305:R804–R810.
Yu N, Chu C, Kunitake T, Kato K, Nakazato M, Kannan H. Cardiovascular actions of central neuropeptide W in conscious rats. Regul Pept 2007;138:82–86.
Niimi M, Murao K. Neuropeptide W as a stress mediator in the hypothalamus. Endocrine 2005;27:51–54.
Takenoya F, Wang L, Kageyama H et al. Neuropeptide W-induced hypophagia is mediated through corticotropin-releasing hormone-containing neurons. J Mol Neurosci 2015;56:789–798.
Xing Y, Liu Y, Deng M et al. The synergistic effects of opioid and neuropeptide B/W in rat acute inflammatory and neuropathic pain models. Eur J Pharmacol 2021;898:173979.
Yamamoto T, Saito O, Shono K, Tanabe S. Anti-hyperalgesic effects of intrathecally administered neuropeptide W-23, and neuropeptide B, in tests of inflammatory pain in rats. Brain Res 2005;1045:97–106.
Atici AE, Arabacı Tamer S, Levent HN et al. Neuropeptide W attenuates oxidative multi-organ injury in rats induced with intra-abdominal sepsis. Inflammation 2022;45:279–296.
Arabacı Tamer S, Akbulut S, Peker Eyüboğlu İ et al. Peripheral administration of Neuropeptide-W protects against stress-induced gastric injury in rats. Life Sci 2022;310:121087.
Koyuncuoglu T, Ipek BE, Dertsiz EK, Akakin D, Yuksel M, Yegen BC, The neuroprotective effects of neuropeptide W in newborn rats with cerebral palsy, Acta Physiologica, Wiley, 2019, pp. 87–87.
Tamer SA, Akbulut S, Akakin D, Yegen BC, Protective effects of neuropeptide-W on stress-induced gastric ulcer in rats, Acta Physiologica, Wiley, 2019, pp. 116–116.
Özdemir ZN, Gökhan T, Pınar K et al. Nicotine alleviates colitis-induced damage in rats via its anti-oxidative activity. Marmara Med J 2014;27:13–20.
Ahn H, Lindhagen J, Lundgren O. Measurement of colonic blood flow with laser Doppler flowmetry. Scand J Gastroenterol 1986;21:871–880.
Arabacı Tamer S, Üçem S, Büke B et al. Regular moderate exercise alleviates gastric oxidative damage in rats via the contribution of oxytocin receptors. J Physiol 2020;598:2355–2370.
Cooper HS, Murthy S, Shah R, Sedergran D. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 1993;69:238–249.
Farghaly HS, Thabit RH. l-Arginine and aminoguanidine reduce colonic damage of acetic acid-induced colitis in rats: Potential modulation of nuclear factor-κB/p65. Clin Exp Pharmacol Physiol 2014;41:769–779.
Tuğtepe H, Şener G, Bıyıklı NK et al. The protective effect of oxytocin on renal ischemia/reperfusion injury in rats. Regul Pept 2007;140:101–108.
Arabacı Tamer S, Yıldırım A, Arabacı Ş et al. Treatment with estrogen receptor agonist ERβ improves torsion-induced oxidative testis injury in rats. Life Sci 2019;222:203–211.
Gue M, Bonbonne C, Fioramonti J et al. Stress-induced enhancement of colitis in rats: CRF and arginine vasopressin are not involved. Am J Physiol Gastrointest Liver Physiol 1997;272:G84–G91.
Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011;474:307–317.
Dembiński A, Warzecha Z, Ceranowicz P et al. Synergic interaction of rifaximin and mutaflor (Escherichia coli Nissle 1917) in the treatment of acetic acid-induced colitis in rats. Gastroenterol Res Pract 2016;2016:3126280.
Jurjus AR, Khoury NN, Reimund J-M. Animal models of inflammatory bowel disease. J Pharmacol Toxicol Methods 2004;50:81–92.
Randhawa PK, Singh K, Singh N, Jaggi AS. A review on chemical-induced inflammatory bowel disease models in rodents. Kor J Physiol Pharmacol 2014;18:279–288.
Rodríguez Basso A, Carranza A, Zainutti VM, Bach H, Gorzalczany SB. Pharmacological activity of peperina (Minthostachys verticillata) on gastrointestinal tract. J Ethnopharmacol 2021;269:113712.
Essel LB, Obiri DD, Osafo N, Antwi AO, Duduyemi MB. Ulcerative colitis induced with acetic acid is ameliorated by Antrocaryon micraster through reduced serum levels of tumor necrosis factor alpha and interleukin‑6 in Sprague Dawley rats. Pharmacognosy Res 2020;12.
Ahmed O, Farid A, Elamir A. Dual role of melatonin as an anti-colitis and anti-extra intestinal alterations against acetic acid-induced colitis model in rats. Sci Rep 2022;12:6344.
Carty E, De Brabander M, Feakins RM, Rampton DS. Measurement of in vivo rectal mucosal cytokine and eicosanoid production in ulcerative colitis using filter paper. Gut 2000;46:487–492.
El-Akabawy G, El-Sherif NM. Zeaxanthin exerts protective effects on acetic acid-induced colitis in rats via modulation of pro-inflammatory cytokines and oxidative stress. Biomed Pharmacother 2019;111:841–851.
Ordás I, Mould DR, Feagan BG, Sandborn WJ. Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther 2012;91:635–646.
Esworthy RS, Aranda R, Martín MG, Doroshow JH, Binder SW, Chu FF. Mice with combined disruption of Gpx1 and Gpx2 genes have colitis. Am J Physiol Gastrointest Liver Physiol 2001;281:G848–G855.
Kruidenier L, van Meeteren ME, Kuiper I et al. Attenuated mild colonic inflammation and improved survival from severe DSS-colitis of transgenic Cu/Zn-SOD mice. Free Radic Biol Med 2003;34:753–765.
Çevik Ö, Şener A, Kumral ZÖ et al. Protective and therapeutic effects of Polygonum cognatum Meissn aqueous extract in experimental colitis. Marmara Pharmaceut J 2014;18:126–134.
Motawea MH, Abd Elmaksoud HA, Elharrif MG, Desoky AAE, Ibrahimi A. Evaluation of anti-inflammatory and antioxidant profile of oleuropein in experimentally induced ulcerative colitis. Int J Mol Cell Med 2020;9:224.
Chami B, Ahmad G, Schroder A, San Gabriel P, Witting P. the role of myeloperoxidase and neutrophil extracellular traps in the pathogenesis of inflammatory bowel disease. Gastroenterology 2021;160:S5–S6.
Hansberry DR, Shah K, Agarwal P, Agarwal N, Fecal myeloperoxidase as a biomarker for inflammatory bowel disease. Cureus 2017;9.
Ahmad G, Chami B, Liu Y et al. The synthetic myeloperoxidase inhibitor AZD3241 ameliorates dextran sodium sulfate stimulated experimental colitis. Front Pharmacol 2020;11:556020.
Ando H, Ukena K, Nagata S, Handbook of hormones: comparative endocrinology for basic and clinical research, Academic 2021.
Hochol A, Belloni AS, Rucinski M et al. Expression of neuropeptides B and W and their receptors in endocrine glands of the rat. Int J Mol Med 2006;18:1101–1106.
Saijo H, Tatsumi N, Arihiro S et al. Microangiopathy triggers, and inducible nitric oxide synthase exacerbates dextran sulfate sodium-induced colitis. Lab Invest 2015;95:728–748.
Hultén L, Lindhagen J, Lundgren O, Fasth S, Ahrén C. Regional intestinal blood flow in ulcerative colitis and Crohn’s disease. Gastroenterology 1977;72:388–396.
Leung FW, Koo A. Mucosal vascular stasis precedes loss of viability of endothelial cells in rat acetic acid colitis. Dig Dis Sci 1991;36:727–732.
Chottova Dvorakova M. Distribution and function of neuropeptides W/B signaling system. Front Physiol 2018;9:981.
Ferrer R, Moreno JJ. Role of eicosanoids on intestinal epithelial homeostasis. Biochem Pharmacol 2010;80:431–438.
Le Loupp AG, Bach-Ngohou K, Bourreille A et al. Activation of the prostaglandin D2 metabolic pathway in Crohn’s disease: involvement of the enteric nervous system. BMC Gastroenterol 2015;15:112.
Melgar S, Drmotova M, Rehnström E, Jansson L, Michaëlsson E. Local production of chemokines and prostaglandin E2 in the acute, chronic and recovery phase of murine experimental colitis. Cytokine 2006;35:275–283.
Vong L, Ferraz JG, Panaccione R, Beck PL, Wallace JL. A pro-resolution mediator, prostaglandin D(2), is specifically up-regulated in individuals in long-term remission from ulcerative colitis. Proc Natl Acad Sci USA 2010;107:12023–12027.
Hanauer SB, Sandborn W. Management of Crohn’s disease in adults. Am J Gastroenterol 2001;96:635–643.
Kefalakes H, Stylianides TJ, Amanakis G, Kolios G. Exacerbation of inflammatory bowel diseases associated with the use of nonsteroidal anti-inflammatory drugs: myth or reality? Eur J Clin Pharmacol 2009;65:963–970.
Singh VP, Patil CS, Jain NK, Kulkarni SK. Aggravation of inflammatory bowel disease by cyclooxygenase-2 inhibitors in rats. Pharmacology 2004;72:77–84.
Wang D, DuBois RN. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene 2010;29:781–788.
Aryannejad A, Tabary M, Noroozi N et al. Anti-inflammatory effects of ivermectin in the treatment of acetic acid-induced colitis in rats: involvement of GABA(B) receptors. Dig Dis Sci 2022;67:3672–3682.
Camacho-Barquero L, Villegas I, Sánchez-Calvo JM et al. Curcumin, a Curcuma longa constituent, acts on MAPK p38 pathway modulating COX-2 and iNOS expression in chronic experimental colitis. Int Immunopharmacol 2007;7:333–342.
Dudhgaonkar SP, Tandan SK, Kumar D, Raviprakash V, Kataria M. Influence of simultaneous inhibition of cyclooxygenase-2 and inducible nitric oxide synthase in experimental colitis in rats. Inflammopharmacology 2007;15:188–195.
El-Medany A, Mahgoub A, Mustafa A, Arafa M, Morsi M. The effects of selective cyclooxygenase-2 inhibitors, celecoxib and rofecoxib, on experimental colitis induced by acetic acid in rats. Eur J Pharmacol 2005;507:291–299.
Lee Y, Kim W, Hong S et al. Colon-targeted celecoxib ameliorates TNBS-induced rat colitis: a potential pharmacologic mechanism and therapeutic advantages. Eur J Pharmacol 2014;726:49–56.
Kjærgaard S, Damm MMB, Chang J, et al. Altered structural expression and enzymatic activity parameters in quiescent ulcerative colitis: are these potential normalization criteria? Int J Mol Sci 2020;21.
Zielińska AK, Sałaga M, Siwiński P, Włodarczyk M, Dziki A, Fichna J. Oxidative stress does not influence subjective pain sensation in inflammatory bowel disease patients. Antioxidants (Basel) 2021;10.
Olén O, Erichsen R, Sachs MC et al. Colorectal cancer in ulcerative colitis: a Scandinavian population-based cohort study. Lancet 2020;395:123–131.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arabacı Tamer, S., Akbulut, S., Erdoğan, Ö. et al. Neuropeptide W Exhibits Preventive and Therapeutic Effects on Acetic Acid-Induced Colitis via Modulation of the Cyclooxygenase Enzyme System. Dig Dis Sci 68, 2441–2453 (2023). https://doi.org/10.1007/s10620-022-07811-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-022-07811-2