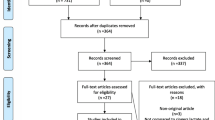
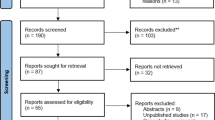
Abstract
Background
We aimed to compare outcomes according to a Lactated Ringers (LR) versus Normal Saline (NS)-based strategy for acute pancreatitis.
Methods
A database search through November 2020 was done to identify studies comparing LR to NS for fluid rehydration in AP. The primary endpoint was systemic inflammatory response syndrome (SIRS) at 24 h. Mantel–Haenszel pooled odds ratios (OR) and 95% confidence intervals were constructed using a random effects model. Heterogeneity was assessed using the I2 statistic. Publication bias was assessed using funnel plots.
Results
Six studies were included totaling 549 patients. No difference in the odds of developing SIRS was noted at 24 h (pooled OR 0.59, 95% CI 0.22–1.62, P = 0.31) between LR and NS. I2 indices showed low heterogeneity between the groups, and a funnel plot showed no obvious publication bias. There was no difference between LR and NS found for SIRS at 48 and 72 h, mortality, and other secondary outcomes. LR was associated with a decreased need for ICU admission.
Conclusions
This updated meta-analysis does not support the previously published finding that the use of LR (rather than NS) leads to a statistically significant decreased odds of SIRS in acute pancreatitis.





Similar content being viewed by others
References
Peery AF, Crocket SD, Murphy CC et al. Burden and cost of gastrointestinal, liver and pancreatic diseases in the United States: update 2018. Gastroenterology 2019;156:254-272.e11.
Banks PA, Bollen TL, Dervenis C et al. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102–111.
Sternby H, Bolado F, Canaval-Zuleta HJ, Marra-Lopez C et al. Determinants of severity in acute pancreatitis: a nation-wide multicenter prospective cohort study. Ann Surg 2019;270:348–355.
Garg PK, Singh VP. Organ failure due to systemic injury in acute pancreatitis. Gastroenterology 2019;156:2008–2023.
Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology 2013;13:e1.
Mofidi R, Duff MD, Wigmore SJ, Madhavan KK, Garden OJ, Parks RW. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br J Surg 2006;93:738–744.
Singh VK, Wu BU, Bollen TL et al. Early systemic inflammatory response syndrome is associated with severe acute pancreatitis. Clin Gastroenterol Hepatol 2009;7:1247–1251.
Garcia-Rayado G, Cardenas-Jaen K, de-Madaria E. Towards evidence-based and personalized care of acute pancreatitis. United Eur Gastroenterol J 2020;8:403–409.
Wu BU, Johannes RS, Sun X, Conwell DL, Banks PA. Early changes in blood urea nitrogen predict mortality in acute pancreatitis. Gastroenterology 2009;137:129–135.
Gardner TB, Vege SS, Pearson RK, Chari ST. Fluid resuscitation in acute pancreatitis. Clin Gastroenterol Hepatol 2008;6:1070–1076.
Vege SS, DiMagno MJ, Forsmark CE, Martel M, Barkun AN. Initial medical treatment of acute pancreatitis: American gastroenterological association institute technical review. Gastroenterology 2018;154:1103–1139.
Haydock MD, Mittal A, Wilms HR, Phillips A, Petrov MS, Windsor JA. Fluid therapy in acute pancreatitis: anybody’s guess. Ann Surg 2013;257:182–188.
de-Madaria E, Garg PK. Fluid therapy in acute pancreatitis—aggressive or adequate? Time for reappraisal. Pancreatology 2014;14:433–435.
Iqbal U, Anwar H, Scribani M. Ringer’s lactate versus normal saline in acute pancreatitis: a systematic review and meta-analysis. J Dig Dis 2018;19:335–341. https://doi.org/10.1111/1751-2980.12606
Lee A, Ko C, Buitrago C, Hiramoto B, HIIson L, Buxbaum J. Lactated ringers vs normal saline resuscitation for mild acute pancreatitis: a randomized trial. Gastroenterology 2021;160:955.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. PLoS Med 2009;6:e1000097.
Wells G, Shea B, O’Connel D, et al. Ottawa Hospital Research Institute. Published 2019. Accessed November 15, 2020. http://ohri.ca/programs/clinical_epidemiology/oxford.asp.
Jadad AR, Moore RA, Carrol D et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials 1996;17:1–12.
American College of Chest Physicians, Society of Critical Care Medicine. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions of sepsis and organ failure and guidelines for the use of innovative therapies in sepsis – PubMed. Crit Care Med. 1992;20:864–874. Accessed February 8, 2021. https://pubmed.ncbi.nlm.nih.gov/1597042/.
de-Madaria E, Herrera-Marante I, Gonzalez-Camacho V et al. Fluid resuscitation with lactated Ringer’s solution vs normal saline in acute pancreatitis: a triple-blind, randomized, controlled trial. United Eur Gastroenterol J 2018;6:63–72.
Wu BU, Hwang JQ, Gardner TH et al. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol 2011;9:710–717.
Choosakul S, Harinwan K, Chirapongsathorn S et al. Comparison of normal saline versus lactated Ringer’s solution for fluid resuscitation in patients with mild acute pancreatitis. A randomized controlled trial. Pancreatology 2018;18:507–512.
Aboelsoud MM, Siddique O, Morales A, Seol Y, Al-Qadi MO. Fluid choice matters in critically-ill patients with acute pancreatitis: lactated ringer’s versus isotonic saline. R I Med J 2013;2016:39–42.
Lipinski M, Rydzewska-Rosolowska A, Rydzewski A, Rydzewska G. Fluid resuscitation in acute pancreatitis: normal saline or lactated Ringer’s solution? World J Gastroenterol 2015;21:9367–9372.
Bassi D, Kollias N, Fernandez-del Castillo C, Foitzik T, Warshaw AL, Rattner DW. Impairment of pancreatic microcirculation correlates with the severity of acute experimental pancreatitis. J Am Coll Surg 1994;179:257–263.
de-Madaria E, Banks PA, Moya-Hoyo N, Wu BU, Rey-Riveiro M, Acevedo-Piedra NG. Early factors associated with fluid sequestration and outcomes of patients with acute pancreatitis. Clin Gastroenterol Hepatol 2014;12:997–1002.
Kinnala PJ, Kuttila KT, Gronroos JM, Havia TV, Nevalainen TJ, Niinikoski JH. Pancreatic tissue perfusion in experimental acute pancreatitis. Eur J Surg 2001;167:689–694.
D’Haese J, Werner J. Translational research for acute pancreatitis—which results have really influenced our therapy? Visc Med 2018;34:436–438.
Radadiya D, Devani K, Arora S et al. Peri-procedural aggressive hydration for post endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis prophylaxis: meta-analysis of randomized controlled trials. Pancreatology 2019;19:819–827.
Buter A, Imrie CW, Carter CR, Evans S, McKay CJ. Dynamic nature of early organ dysfunction determines outcome in acute pancreatitis. Br J Surg 2002;89:298–302.
Johnson CD, Abu-Hilal M. Persistent organ failure during the first week as a marker of fatal outcome in acute pancreatitis. Gut 2004;53:1340–1344.
Yang CJ, Chne J, Phillips ARJ, Windsor JA, Petrov MS. Predictors of severe and critical acute pancreatitis: a systematic review. Dig Liver Dis 2014;46:446–451.
Rodriguez JR, Razo AO, Targarona J, Thayer SP, Rattner DW, Warshaw AL. Debdridement and closed packing for sterile or infected necrotizing pancreatitis: insights into indications and outcomes in 167 patients. Ann Surg 2008;247:294–299.
Rattner DW, Legermate DA, Lee MJ, Mueller PR, Warshaw AL. Early surgical debridement of symptomatic pancreatic necrosis is beneficial irrespective of infection. Am J Surg 1992;163:105–110.
Bhoomagoud M, Jung T, Atladottir J et al. Reducing extracellular pH sensitizes the acinar cell to secreatogogue-induced pancreatitis responses in rats. Gastroenterology 2009;137:1083–1092.
Noble MD, Romac J, Vigna SR, Liddle RA. A pH-sensitive, neurogenic pathway mediates disease severity in a model of post-ERCP pancreatitis. Gut 2008;57:1566–1571.
Iraporda C, Errea A, Romanin DE, Cayet D, Pereyra E, Pignataro O. Lactate and short chain fatty acids produced by microbial fermentation downregulate proinflammatory responses in intestinal epithelial cells and myeloid cells. Immunobiology 2015;220:1161–1169.
Khatua B, Yaron JR, El-Kurdi B, Kostenko S, Papachristou GI, Singh VP. Ringer’s lactate prevents early organ failure by providing extracellular calcium. J Clin Med 2020;9:263.
Gheorghe C, Dadu R, Blot C et al. Hyperchloremic metabolic acidosis following resuscitation of shock. Chest 2010;138:1521–1522. https://doi.org/10.1378/chest.10-1458.
Krajewski ML, Raghunathan K, Paluszkiewicz SM, Schermer CR, Shaw AD. Meta-analysis of high- versus low-chloride content in perioperative and critical care fluid resuscitation. Br J Surg 2015;102:24–36.
Rajamaki K, Nordstrom T, Nurmi K et al. Extracellular acidosis is a novel danger signal alerting innate immunity via the NLRP3 inflammasome. J Biol Chem 2013;288:13410–13419.
Hadimioglu N, Saadawy I, Saglam T, Ertug Z, Dinckan A. The effect of different crystalloid solutions on acid-base balance and early kidney function after kidney transplantation. Anesth Analg 2008;107:264–269.
Kellum JA, Song M, Li J. Science review: extracellular acidosis and the immune response: clinical and physiologic implications. Crit Care 2004;8:331–336.
Raghunathan K, Shaw A, Nathanson B et al. Association between the choice of IV crystalloid and in-hospital mortality among critically ill adults with sepsis*. Crit Care Med 2014;42:1585–1591.
Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA 2012;308:1566–1572.
Funding
There was no grant support involved in the conduction, research, or writing of this manuscript. There authors disclose no financial arrangements related to the research or assistance with manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest for this article.
Informed consent
For this type of study (retrospective meta-analysis), formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Figure 1. Forrest plot of LR vs NS for moderate-severe pancreatitis incidence. Pooled OR 0.86, 95% CI 0.40–1.87, P = 0.71.

Supplementary Figure 2. Forrest plot of LR versus NS for organ failure incidence. Pooled OR 0.69, 95% CI 0.29–1.63, P = 0.40.

Supplementary Figure 3. Forrest plot of LR versus NS for local complication incidence. Pooled OR 0.48, 95% CI 0.20–1.16, P= 0.10.

Supplementary Figure 4. Forrest plot of LR versus NS for incidence of pancreatic necrosis. Pooled OR 0.65, 95% CI 0.23–1.81, P= 0.41.

Supplementary Figure 5. Forrest plot of LR versus NS for need for invasive treatment. Pooled OR 0.66, 95% CI 0.08–5.61, P= 0.70.

Supplementary Figure 6. Forrest plot of LR versus NS for need for nutritional support. Pooled OR 1.26, 95% CI 0.08–20.45, P = 0.87.

Rights and permissions
About this article
Cite this article
Vedantam, S., Tehami, N., de-Madaria, E. et al. Lactated Ringers Does Not Reduce SIRS in Acute Pancreatitis Compared to Normal Saline: An Updated Meta-Analysis. Dig Dis Sci 67, 3265–3274 (2022). https://doi.org/10.1007/s10620-021-07153-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-021-07153-5