Abstract
Background
Direct-acting antivirals (DAAs) are current standard of HCV treatment (Rx). However, data remain lacking on real-world safety, patterns of biochemical, virologic responses, and sustained virologic response (SVR12) rate in geriatric patients.
Aims
The present study assessed clinical presentation, safety, SVR12 rate, dynamic changes in HCV RNA, ALT, and AFP in geriatric patients (age ≥ 65 year old, G1) versus non-geriatric patients (G2) with chronic hepatitis C and received DAA treatment.
Methods
This was a single-center, retrospective study on 183 patients with DAA Rx and 12-week post-Rx follow-up.
Results
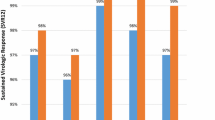
There were no significant differences in patterns of biochemical and virologic responses between the two groups. Undetectable HCV RNA rates were 67.2% versus 75.7% (p = 0.22) and 77.3% versus 84.3% (p = 0.24) at Rx week 2 and Rx week 4, respectively. The SVR12 rate was comparable in 2 groups, 94.1% (G1) versus 95.7% (G2, p = 0.64). ALT normalization rates were 91.2% versus 91.3% (p = 0.98), 92.6% versus 93.9% (p = 0.74), and 97.1% versus 97.4% (p = 0.89) at Rx week 2, post-Rx week12, and post-Rx week 24, respectively. AFP normalization was lower in G1 with 89.7% versus 95.7% (p = 0.12), 77.9% versus 87.8% (p = 0.08), and 79.4% versus 92.2% (p = 0.01), at Rx week 2, and post-Rx week 12, and post-Rx week 24, respectively. Both groups showed similar side effects profile including fatigue 11.8% versus 12.2% (p = 0.93) and headache 11.8% versus 13.9% (p = 0.68).
Conclusion
Based on our real-world data, geriatric patients had excellent and comparable treatment outcomes with non-geriatric patients in safety and SVR12 rates to different DAA regimens.



Similar content being viewed by others
References
Gower E, Estes C, Blach E, et al. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45–S57.
Edlin BR, Eckhardt BJ, Shu MA, et al. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology. 2015;62:1353–1363.
Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714.
Thabut E, Le Calvez S, Thibault V, et al. Hepatitis C in 6865 patients 65 year or older: a severe and neglected curable disease? Am J Gastroenterol. 2006;101:1260–1267.
Poynard T, Ratziu V, Charlotte F, et al. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis C. J Hepatol. 2001;34:730–739.
Pradat P, Voirin N, Tillmann HL, et al. Progression to cirrhosis in hepatitis C patients: an age-dependent process. Liver Int. 2007;27:335–339.
Ben Yehuda A, Globerson A, Krichevsky S, et al. Ageing and the mis-match repair system. Mech Ageing Dev. 2000;121:173–179.
Morgan TR, Ghany MG, Kim HY, et al. Outcome of sustained virological response with histological advanced chronic hepatitis. Hepatology. 2010;52:833–844.
Backus LI, Boothroyd DB, Phillips BR, et al. A sustained virologic response reduce risk of all cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509–516.
Huang CF, Yeh ML, Huang CL, et al. Risk of hepatitis C virus related hepatocellular carcinoma between subjects with spontaneous and treatment induced viral clearance. Oncotarget. 2017;8:43925–43933.
Huang CF, Yu ML. Treating hepatitis C in the elderly: pharmacotherapeutic considerations and developments. Expert Opin Pharmacother. 2017;18:1867–1874.
Gramenzi A, Conti F, Felline F, et al. Hepatitis C virus-related chronic liver disease in elderly patients: an Italian cross-sectional study. J Viral Hepat. 2009;17:360–366.
Beeste LA, Leipertz SL, Green PK, et al. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001–2013. Gastroenterology. 2015;149:1471–1482.
Ermis F, Senocak TE. New treatment strategies for hepatitis C infection. World J Hepatol. 2015;7:2100–2109.
Huynh T, Zhang J, Hu KQ. Hepatitis C virus clearance by direct acting antiviral results in rapid resolution of hepatocytic injury as indicated by both alanine aminotransferase and aspartate aminotransferase normalization. J Clin Transl Hepatol. 2018;6:258–263.
Huynh T, Hu KQ. Direct acting antiviral-induced dynamic reduction of serum alpha fetoprotein in hepatitis C patients without hepatocellular carcinoma. Front Med. 2019;13:658–666.
American Association for the Study of Liver Disease/Infectious Diseases Society of America. 2017. Recommendations for testing, management, and treating hepatitis C.
Surjadi M. Chronic hepatitis C screening, evaluation, and treatment update in the age of direct-acting antiviral. Workplace Health Saf. 2018;66:302–309.
Ozono Y, Nagata K, Hasuike S, et al. Efficacy and safety of sofosbuvir and ledipasvir in Japanese patients aged 75 years or over with hepatitis C genotype 1. World J Hepatol. 2017;9:1340–1345.
Saab S, Park SH, Mizokami M, et al. Safety and efficacy of ledipasvir/sofosbuvir for the treatment of genotype 1 hepatitis C in subjects aged 65 years or older. Hepatology. 2016;63:1112–1119.
Trifan A, Stanciu C, Gheorghe L, et al. Efficacy and safety of paritaprevir/ritonavir, ombitasvir, and dasabuvir with ribavirin for the treatment of HCV genotype 1b compensated cirrhosis in patients age 70 years or older. Medicine. 2017;96:50.
Ascione A, DeLuca M, Melazzini M, et al. Safety and efficacy of ombitasvir/paritaprevir/ritonavir/dasabuvir plus ribavirin in patients over 65 years with HCV genotype 1 cirrhosis. Infection. 2018;46:607–615.
Elbaz T, Abdo M, Omar H, et al. Efficacy and safety of sofosbuvir and daclatasvir with or without ribavirin in elderly patients with chronic hepatitis C virus infection. J Med Virol. 2019;91:272–277.
Dultz G, Muller T, Petersen J, et al. Effectiveness and safety of direct acting antiviral combination therapies for treatment of hepatitis C virus in elderly patients: results from the Geman Hepatitis C registry. Drugs Aging. 2018;35:843–857.
Conti F, Brillanti S, Buonfiglioli F, et al. Safety and efficacy of direct acting antivirals for the treatment of chronic hepatitis C in a real world population age 65 years and older. J Viral Hepat. 2017;24:454–463.
Knodell RG, Ishak KG, Black WC, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–435.
Desmet VJ, Gerber M, Hoofnagle JH, et al. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–1520.
Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003–2010. Ann Intern Med. 2014;160:293–300.
Baati N, Silverman AL, Gordon SC. Serum alpha-fetoprotein levels and liver histology in patients with chronic hepatitis C. Am J Gastroenterol. 1998;93:2452–2456.
Chu CW, Hwang SJ, Luo JC, et al. Clinical, virologic, and pathologic significance of elevated serum alpha-fetoprotein levels in patients with chronic hepatitis C. J Clin Gastroenterol. 2001;32:240–244.
Hu KQ, Kyulo NL, Lim N, et al. Clinical significance of elevated alpha-fetoprotein in patients with chronic hepatitis C, but not hepatocellular carcinoma. Am J Gastroenterol. 2004;99:860–865.
Sherman M. Hepatocellular carcinoma: epidemiology, risk factors, and screening. Semin Liver Dis. 2005;25:143–154.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Tung Huynh and Ke-Qin Hu declare that they have no conflict of interest related to this study. This study was an investigator-initiated study, and no external funding in any source has been obtained. Tung Huynh has nothing to disclose. Dr. Hu is on speaker bureau for Gilead Sciences.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huynh, T., Hu, KQ. Excellent Safety and Sustained Virologic Response to Direct-Acting Antivirals Treatment in HCV-Infected Geriatric Patients: A Real-World Data. Dig Dis Sci 66, 1327–1334 (2021). https://doi.org/10.1007/s10620-020-06286-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06286-3