Abstract
Background
Diet is suggested to participate in the etiology of inflammatory bowel diseases (IBD). Repeated exposure to Maillard reaction products (MRPs), molecules resulting from reduction reactions between amino acids and sugars during food heating, has been reported to be either potentially detrimental or beneficial to health.
Aims
The aim of this study is to determine the effect of repeated oral ingestion of N ε-carboxymethyllysine (CML), an advanced MRP, on the onset of two models of experimental IBD and on the gut microbiota composition of mice.
Methods
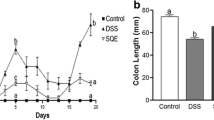
Mice received either saline (control) or N ε-carboxymethyllysine daily for 21 days. For the last week of treatment, each group was split into subgroups, receiving dextran sulfate sodium salt (DSS) or trinitrobenzenesulfonic acid (TNBS) to induce colitis. Intensity of inflammation was quantified, and cecal microbiota characterized by bacterial 16S ribosomal RNA (rRNA) amplicon sequencing.
Results
Daily oral administration of N ε-carboxymethyllysine did not induce intestinal inflammation and had limited impact on gut microbiota composition (Bacteroidaceae increase, Lachnospiraceae decrease). DSS and TNBS administration resulted in expected moderate experimental colitis with a shift of Bacteroidetes/Firmicutes ratio and a significant Proteobacteria increase but with distinct profiles: different Proteobacteria taxa for DSS, but mainly Enterobacteriaceae for TNBS. While N ε-carboxymethyllysine exposure failed to prevent the inflammatory response, it allowed maintenance of healthy gut microbiota profiles in mice treated with DSS (but not TNBS).
Conclusions
Repeated oral exposure to CML limits dysbiosis in experimental colitis. IBD patients may modulate their microbiota profile by regulating the level and type of dietary MRP consumption.







Similar content being viewed by others
References
Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712.
Everard A, Cani PD. Diabetes, obesity and gut microbiota. Best Pract Res Clin Gastroenterol. 2013;27:73–83.
Flint HJ, Scott KP, Louis P, Duncan SH. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol. 2012;9:577–589.
Carbonero F, Benefiel AC, Alizadeh-Ghamsari AH, Gaskins HR. Microbial pathways in colonic sulfur metabolism and links with health and disease. Front Physiol. 2012;3:448. doi:10.3389/fphys.2012.00448.
Ou J, Carbonero F, Zoetendal EG, et al. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr. 2013;98:111–120. doi:10.3945/ajcn.112.056689.
O’Keefe SJ, Li JV, Lahti L, et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat Commun. 2015;6:6342.
Gomez A, Petrzelkova KJ, Burns MB, et al. Gut microbiome of coexisting BaAka pygmies and bantu reflects gradients of traditional subsistence patterns. Cell Rep. 2016;14:2142–2153.
Shen W, Wolf PG, Carbonero F, et al. Intestinal and systemic inflammatory responses are positively associated with sulfidogenic bacteria abundance in high-fat-fed male C57BL/6J mice. J Nutr. 2014;144:1181–1187. doi:10.3945/jn.114.194332.
Devkota S, Wang Y, Musch MW, et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10–/– mice. Nature. 2012;487:104–108.
He Q, Li X, Liu C, et al. Dysbiosis of the fecal microbiota in the TNBS-induced Crohn’s disease mouse model. Appl Microbiol Biotechnol. 2016;100:4485–4494.
Gkouskou KK, Deligianni C, Tsatsanis C, Eliopoulos AG. The gut microbiota in mouse models of inflammatory bowel disease. Front Cell Infect Microbiol. 2014;4:28. doi:10.3389/fcimb.2014.00028.
Munyaka PM, Rabbi MF, Khafipour E, Ghia J. Acute dextran sulfate sodium (DSS)-induced colitis promotes gut microbial dysbiosis in mice. J Basic Microbiol. 2016;56:986–998.
Pabby V, Friedman S. Diet affects symptoms and medication response in inflammatory bowel disease. Dig Dis Sci. 2013;58:1173–1174. doi:10.1007/s10620-013-2619-8.
Wautier MP, Tessier FJ, Wautier JL. Advanced glycation end products: a risk factor for human health. Ann Pharm Fr. 2014;72:400–408. doi:10.1016/j.pharma.2014.05.002.
de Oliveira FC, Coimbra JSR, de Oliveira EB, Zuñiga ADG, Rojas EEG. Food protein–polysaccharide conjugates obtained via the Maillard reaction: a review. Crit Rev Food Sci Nutr. 2016;56:1108–1125.
Goldberg T, Cai W, Peppa M, et al. Advanced glycoxidation end products in commonly consumed foods. J Am Diet Assoc. 2004;104:1287–1291.
Fu MX, Requena JR, Jenkins AJ, Lyons TJ, Baynes JW, Thorpe SR. The advanced glycation end product, nepsilon-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J Biol Chem. 1996;271:9982–9986.
Macías-Cervantes MH, Rodríguez-Soto JMD, Uribarri J, Díaz-Cisneros FJ, Cai W, Garay-Sevilla ME. Effect of an advanced glycation end product-restricted diet and exercise on metabolic parameters in adult overweight men. Nutrition. 2015;31:446–451.
Roncero-Ramos I, Delgado-Andrade C, Tessier FJ, et al. Metabolic transit of n ε-carboxymethyl-lysine after consumption of AGEs from bread crust. Food Funct. 2013;4:1032–1039.
Schleicher ED, Wagner E, Nerlich AG. Increased accumulation of the glycoxidation product N(epsilon)-(carboxymethyl)lysine in human tissues in diabetes and aging. J Clin Invest. 1997;99:457–468. doi:10.1172/JCI119180.
Jara N, Leal M, Bunout D, et al. Dietary intake increases serum levels of carboxymethil-lysine (CML) in diabetic patients. Nutr Hosp. 2012;27:1272–1278.
Llaurado G, Ceperuelo-Mallafre V, Vilardell C, et al. Advanced glycation end products are associated with arterial stiffness in type 1 diabetes. J Endocrinol. 2014;221:405–413. doi:10.1530/JOE-13-0407.
Mishra N, Saxena S, Shukla RK, et al. Association of serum N ε-carboxy methyl lysine with severity of diabetic retinopathy. J Diabetes Complicat. 2016;30:511–517.
Van Eupen MG, Schram MT, Colhoun HM, Scheijen JL, Stehouwer CD, Schalkwijk CG. Plasma levels of advanced glycation endproducts are associated with type 1 diabetes and coronary artery calcification. Cardiovasc Diabetol. 2013;12:149.
Dammann P, Sell DR, Begall S, Strauch C, Monnier VM. Advanced glycation end-products as markers of aging and longevity in the long-lived Ansell’s mole-rat (Fukomys anselli). J Gerontol Ser A Biomed Sci Med Sci. 2011;67:573–583.
Van Puyvelde K, Mets T, Njemini R, Beyer I, Bautmans I. Effect of advanced glycation end product intake on inflammation and aging: a systematic review. Nutr Rev. 2014;72:638–650.
Whitson HE, Arnold AM, Yee LM, et al. Serum carboxymethyl-lysine, disability, and frailty in older persons: the cardiovascular health study. J Gerontol Ser A Biomed Sci Med Sci. 2013;69:710–716.
Poulsen MW, Bak MJ, Andersen JM, et al. Effect of dietary advanced glycation end products on postprandial appetite, inflammation, and endothelial activation in healthy overweight individuals. Eur J Nutr. 2014;53:661–672.
Semba RD, Gebauer SK, Baer DJ, et al. Dietary intake of advanced glycation end products did not affect endothelial function and inflammation in healthy adults in a randomized controlled trial. J Nutr. 2014;144:1037–1042. doi:10.3945/jn.113.189480.
Gaens KH, Ferreira I, van de Waarenburg MP, et al. Protein-bound plasma nepsilon-(carboxymethyl)lysine is inversely associated with central obesity and inflammation and significantly explain a part of the central obesity-related increase in inflammation: the Hoorn and CODAM studies. Arterioscler Thromb Vasc Biol. 2015;35:2707–2713. doi:10.1161/ATVBAHA.115.306106.
Delgado-Andrade C. Carboxymethyl-lysine: thirty years of investigation in the field of AGE formation. Food Funct. 2016;7:46–57.
Tessier FJ, Niquet-Léridon C, Jacolot P, et al. Quantitative assessment of organ distribution of dietary protein-bound 13C-labeled nɛ-carboxymethyllysine after a chronic oral exposure in mice. Mol Nutr Food Res. 2016;60:2446–2456.
Tuohy KM, Hinton DJ, Davies SJ, Crabbe MJC, Gibson GR, Ames JM. Metabolism of Maillard reaction products by the human gut microbiota—implications for health. Mol Nutr Food Res. 2006;50:847–857.
Hellwig M, Bunzel D, Huch M, Franz CM, Kulling SE, Henle T. Stability of individual Maillard reaction products in the presence of the human colonic microbiota. J Agric Food Chem. 2015;63:6723–6730.
Helou C, Marier D, Jacolot P, et al. Microorganisms and Maillard reaction products: a review of the literature and recent findings. Amino Acids. 2014;46:267–277.
Helou C, Denis S, Spatz M, et al. Insights into bread melanoidins: fate in the upper digestive tract and impact on the gut microbiota using in vitro systems. Food Funct. 2015;6:3737–3745.
Seiquer I, Rubio LA, Peinado MJ, Delgado-Andrade C, Navarro MP. Maillard reaction products modulate gut microbiota composition in adolescents. Mol Nutr Food Res. 2014;58:1552–1560.
Anton PM, Craus A, Niquet-Léridon C, Tessier FJ. Highly heated food rich in Maillard reaction products limit an experimental colitis in mice. Food Funct. 2012;3:941–949.
Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803.
Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702.
Chassaing B, Aitken JD, Malleshappa M, Vijay‐Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2014:15.25.1–15.25.14.
Kato Y. Neutrophil myeloperoxidase and its substrates: formation of specific markers and reactive compounds during inflammation. J Clin Biochem Nutr. 2016;58:99–104. doi:10.3164/jcbn.15-104.
Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209.
Ettreiki C, Gadonna-Widehem P, Mangin I, Coeffier M, Delayre-Orthez C, Anton PM. Juvenile ferric iron prevents microbiota dysbiosis and colitis in adult rodents. World J Gastroenterol. 2012;18:2619–2629. doi:10.3748/wjg.v18.i21.2619.
Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol. 2013;79:5112–5120. doi:10.1128/AEM.01043-13.
Kuczynski J, Stombaugh J, Walters WA, González A, Caporaso JG, Knight R. Using QIIME to analyze 16S rRNA gene sequences from microbial communities. Curr Protoc Microbiol. 2012;Chapter 1:Unit 1E.5.
Henle T. Dietary advanced glycation end products—a risk to human health? A call for an interdisciplinary debate. Mol Nutr Food Res. 2007;51:1075–1078.
Thornalley PJ. Dietary AGEs and ALEs and risk to human health by their interaction with the receptor for advanced glycation endproducts (RAGE)—an introduction. Mol Nutr Food Res. 2007;51:1107–1110.
Angoorani P, Ejtahed H, Mirmiran P, Mirzaei S, Azizi F. Dietary consumption of advanced glycation end products and risk of metabolic syndrome. Int J Food Sci Nutr. 2016;67:170–176.
Davis KE, Prasad C, Vijayagopal P, Juma S, Imrhan V. Advanced glycation end products, inflammation, and chronic metabolic diseases: links in a chain? Crit Rev Food Sci Nutr. 2016;56:989–998.
Ames JM. Evidence against dietary advanced glycation endproducts being a risk to human health. Mol Nutr Food Res. 2007;51:1085–1090.
Šebeková K, Somoza V. Dietary advanced glycation endproducts (AGEs) and their health effects—PRO. Mol Nutr Food Res. 2007;51:1079–1084.
Li M, Zeng M, He Z, et al. Effects of long-term exposure to free N ε-(carboxymethyl) lysine on rats fed a high-fat diet. J Agric Food Chem. 2015;63:10995–11001.
Hernandez-Hernandez O, Sanz ML, Kolida S, Rastall RA, Moreno FJ. In vitro fermentation by human gut bacteria of proteolytically digested caseinomacropeptide nonenzymatically glycosylated with prebiotic carbohydrates. J Agric Food Chem. 2011;59:11949–11955.
Reichardt N, Gniechwitz D, Steinhart H, Bunzel M, Blaut M. Characterization of high molecular weight coffee fractions and their fermentation by human intestinal microbiota. Mol Nutr Food Res. 2009;53:287–299.
Borrelli RC, Fogliano V. Bread crust melanoidins as potential prebiotic ingredients. Mol Nutr Food Res. 2005;49:673–678.
Carbonero F, Benefiel AC, Gaskins HR. Contributions of the microbial hydrogen economy to colonic homeostasis. Nat Rev Gastroenterol Hepatol. 2012;9:504–518.
Packey CD, Sartor RB. Commensal bacteria, traditional and opportunistic pathogens, dysbiosis and bacterial killing in inflammatory bowel diseases. Curr Opin Infect Dis. 2009;22:292–301. doi:10.1097/QCO.0b013e32832a8a5d.
Kang S, Denman SE, Morrison M, et al. Dysbiosis of fecal microbiota in Crohn’s disease patients as revealed by a custom phylogenetic microarray. Inflamm Bowel Dis. 2010;16:2034–2042.
Williams KL, Fuller CR, Dieleman LA, et al. Enhanced survival and mucosal repair after dextran sodium sulfate-induced colitis in transgenic mice that overexpress growth hormone. Gastroenterology. 2001;120:925–937.
Jurjus AR, Khoury NN, Reimund J. Animal models of inflammatory bowel disease. J Pharmacol Toxicol Methods. 2004;50:81–92.
Neurath MF, Fuss I, Kelsall BL, Stuber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281–1290.
Berry D, Kuzyk O, Rauch I, et al. Intestinal microbiota signatures associated with inflammation history in mice experiencing recurring colitis. Front Microbiol. 2015;6:1408. doi:10.3389/fmicb.2015.01408.
Mills D, Tuohy K, Booth J, et al. Dietary glycated protein modulates the colonic microbiota towards a more detrimental composition in ulcerative colitis patients and non-ulcerative colitis subjects. J Appl Microbiol. 2008;105:706–714.
Konikoff T, Gophna U. Oscillospira: a central, enigmatic component of the human gut microbiota. Trends Microbiol. 2016;24:523–524.
Acknowledgments
The authors thank Camille Boroch for help in conducting animal experiments. This work is dedicated to the memory of our wonderful colleague, Pr. Abalo Chango, who originated this international cooperative work. This work was supported by the Picardy Regional Council of France (ETAMIN project) and by an Arkansas Biosciences Institute grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All applicable national and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institutions or practice at which the studies were conducted.
Rights and permissions
About this article
Cite this article
ALJahdali, N., Gadonna-Widehem, P., Delayre-Orthez, C. et al. Repeated Oral Exposure to N ε-Carboxymethyllysine, a Maillard Reaction Product, Alleviates Gut Microbiota Dysbiosis in Colitic Mice. Dig Dis Sci 62, 3370–3384 (2017). https://doi.org/10.1007/s10620-017-4767-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-017-4767-8