Abstract
Fructobacillus is a lactic-acid bacterium recently identified in fructose-rich environments. Fructobacillus is also known to exhibit unusual growth characteristics due to an incomplete gene encoding alcohol/acetaldehyde hydrogenase, which results in an imbalance in the nicotinamide adenine mononucleotide (NAD+)/NADN levels. Recently, the addition of d-fructose to the culture medium of Fructobacillus strains increased the intracellular nicotinamide mononucleotide (NMN) content. In the present study, we evaluated the functionality of Fructobacillus that produces high levels of NMN, using one substrain (Fructobacillus fructosus OS-1010). Therefore, in this study, we examined its functionality in the interaction between intestinal cells and muscle cells. The results showed that supernatant derived from intestinal epithelial cells (Caco-2 cells) treated with F. fructosus OS-1010 activated muscle cells (C2C12 cells). Further analysis revealed that Caco-2 cells treated with F. fructosus OS-1010 secreted exosomes known as extracellular vesicles, which activated the muscle cells. Furthermore, pathway analysis of the target genes of miRNA in exosomes revealed that pathways involved in muscle cell activation, including insulin signaling and cardiac muscle regulation, neurotrophic factors, longevity, and anti-aging, can be activated by exosomes. In other words, F. fructosus OS-1010 could activate various cells such as the skin and muscle cells, by secreting functional exosomes from the intestinal tract.
Similar content being viewed by others
Introduction
Fructobacillus is a recently discovered lactic-acid bacterium and unlike conventional lactic-acid bacteria, it is known to grow poorly on glucose and has a preference for oxygen (Endo et al. 2018). Fructobacillus have been isolated from fructose-rich environments, including flowers, fruits, fermented fruits and the guts of insects that feed on fructose-rich plants. Fructobacillus is known to exhibit unusual growth characteristics due to an incomplete gene encoding a bifunctional alcohol/acetaldehyde hydrogenase, which results in an imbalance in the nicotinamide adenine mononucleotide (NAD+)/NADH levels. Interestingly, a recent study reported that strains belonging to the genus Fructobacillus use d-fructose as an electron acceptor in aerobic lactic acid fermentation, and the addition of d-fructose to the culture medium increases the intracellular nicotinamide mononucleotide (NMN) content. Sugiyama et al. (2021) successfully isolated a strain of Fructobacillus with high NMN production. Fructobacillus fructosus OS-1010 used in this study is a substrain that produces high levels of NMN, and the purpose of this study was to explore its functionality.
NAD+ is one of the cofactors involved in redox reactions in living organisms (Imai 2009; Imai and Guarente 2016). It is synthesized intracellularly from nicotinamide (NAM) and NMN via the salvage pathway. In particular, NAMPT, which synthesizes NAD+ from NMN, is the rate-limiting enzyme in this pathway. Recently, a series of reports have focused on its pharmacological effects on various diseases, including cardiovascular disease, Alzheimer’s disease, and diabetes (Yoshino et al. 2011; Poddar et al. 2019). If the amount of intracellular NAD+ can be increased, either directly or via conversion of NMN, it is believed that NAD+ can activate the longevity gene Sirtuins, which acts as a cofactor and may ultimately lead to anti-aging of the organism (Imai and Guarente 2014). NAD+ is known to have such a wide range of functions, and F. fructosus OS-1010, which produces NMN and NAD+, would have additional functions as a lactic acid bacteria in addition to those of NMN it produces.
Previous studies have found that F. fructosus OS-1010 directly acts on myotubular C2C12 cells and activates their mitochondria. In this study, in order to clarify the function of F. fructosus OS-1010 as a food, we tried to examine its indirect function on C2C12 cells via the intestinal cells.
Materials and methods
Cell culture
The human colorectal cancer cell line Caco-2 (ATCC, Manassas, VA, USA) and murine skeletal myoblasts C2C12 (Riken Bioresource Center, Tsukuba, Japan) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Nissui, Tokyo, Japan) containing 10% heat-inactivated fetal bovine serum (FBS, Capricorn Scientific GmbH, Ebsdorfergrund, Germany) at 37 °C in 5% CO2. C2C12 cells (2.0 × 105 cells/mL in a 6-well dish) were cultured for 48 h and the medium was replaced with DMEM containing 2% horse serum (HS) (Thermo Fisher Scientific, Waltham, MA, USA) to induce differentiation. After another 24 h, the medium was replaced with DMEM containing 2% HS. The medium was replaced every 2 days and used for experiments 10 days after the induction of differentiation.
Lactic acid bacteria
Substrain OS-1010 of Fructobacillus fructosus, which produces high levels of NMN and NAD+, was provided by Osaka Soda Co. Ltd. (Osaka, Japan). The OS-1010 was heated at 65 °C for 30 min and subsequently spray-dried. This dead bacterial powder contained 2.7 µmol of NMN and 14.2 μmol of NAD per gram of dried bacterial weight. The supernatant of Caco-2 cells treated with F. fructosus OS-1010 was prepared by centrifugation at 3000×g at 10 min.
Reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR)
RNA was prepared from cells using the High Pure RNA Isolation Kit (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer’s protocol. RT-qPCR was performed using the GoTaq 1-Step RT-PCR System (Promega, Madison, WI, USA) and Thermal Cycler Dice Real Time System TP-800 (Takara, Shiga, Japan). The samples were analyzed in triplicate. The PCR primer sequences used were as follows: mouse β-actin forward primer 5′-TGGCACCCAGCACAATGAA-3′ and reverse primer 5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′; mouse Sirt1 forward primer 5′-GCAGACGTGGTAATGTCCAAACAG-3′ and reverse primer 5′-ACATCTTGGCAGTATTTGTGGTGAA-3′; mouse Tfam forward primer 5′-CATTTATCTATCTGAAAGCTTCC-3′ and reverse primer 5′-CTCTTCCCAAGACTTCATTTC-3′; mouse brain-derived growth factor (Bdnf) forward primer 5′-GTCAAGTTGGGAGCCTGAAATAGTG-3′ and reverse primer 5′-AGGATGCTGGTCCAAGTGGTG-3′; and mouse MyoD forward primer 5′-ATGAGGCCTTCGAGACGCTC-3′ and reverse primer 5′-CAGAGCCTGCAGACCTTCGA-3′. β-actin was used as a housekeeping gene. Samples were normalized and analyzed by the ΔΔCT method (Ishibashi et al. 2023).
Mitochondria
Cells were stained with 250 nM MitoTracker Red CMXRos (Thermo Fischer Scientific) at 37 °C for 30 min and subsequently with 200 nM MitoTracker Green FM (Thermo Fischer Scientific) at 37 °C for 30 min. Finally, cells were stained with Hoechst 33,342 (Dojindo, Kumamoto, Japan) at 37 °C for 30 min. Stained cells were analyzed using an IN Cell Analyzer 2200 (Cytiva, Tokyo, Japan) to quantitatively determine the number, area, and activity of mitochondria, and the images were analyzed using the IN Cell Investigator high-content image analysis software (Cytiva) (Inotsuka et al. 2021).
Exosome isolation and treatment
Firstly, Caco-2 cells (1.4 × 105 cells/mL) were cultured in DMEM containing 10% exosome-depleted FBS (System Bioscience, Mountain View, CA, USA) and 100 µg/mL OS-1010 for 24 h. After culture, the MagCapture Exosome Isolation Kit PS ver. 2 (FUJIFILM Wako Pure Chemical Corp.) was used to isolate exosomes from the medium of Caco-2 cells, according to the manufacturer’s instructions. The amount of exosomes used for each experiment was prepared as a protein equivalent and measured using a MicroBCA Protein Assay Kit (Thermo Fisher Scientific Inc.) (Sugihara et al. 2019; Ogawa et al. 2021).
miRNA microarray assay
The expression profiles of miRNAs in the exosomes were evaluated by microarray analysis using a 3D-Gene Human miRNA Oligo chip (Toray, Kanagawa, Japan). MiRNA preparation and subsequent operations were performed by Kamakura Techno-Sciences, Inc. (Kanagawa, Japan). After global normalization of the miRNA expression levels, we calculated the ratios of each miRNA for comparison between the control and experimental samples. We then established criteria for the regulated miRNA: (upregulated miRNA) ratio ≥ 1.5-fold (Bolstad et al. 2003). The miRNA target genes were predicted using TargetScan (https://www.targetscan.org/vert_80/, accessed February 1, 2023). We then used the tools and data provided by Database for Annotation, Visualization, and Integrated Discovery (DAVID, http://david.abcc.ncifcrf.gov, accessed on February 20, 2023) to identify significantly enriched pathways (Huang et al. 2009a, 2009b; Inotsuka et al. 2021; Ishibashi et al. 2023).
Statistical analysis
All experiments were repeated at least three times, and representative data are shown. The results are shown as the mean ± standard error. Multiple comparisons between groups were performed using a one-way ANOVA with Tukey’s post-hoc test. Statistical significance was defined as p < 0.05 when compared to the control (*p < 0.05; **p < 0.01; ***p < 0.001).
Results
Fructobacillus fructosus strain OS-1010 activates muscle cells via intestinal cells
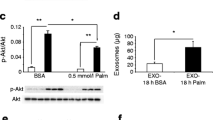
We firstly examined the possibility that Fructobacillus fructosus strain OS-1010 (F. fructosus OS-1010) may activate the interaction between intestinal cells and muscle cells. Therefore, we prepared culture supernatants of OS-1010-treated Caco-2 cells and evaluated whether the addition of supernatants to C2C12 cells, a differentiated myotube cell line, affected their phenotypes. The results showed that the culture supernatant of OS-1010-treated Caco-2 cells induced the expression of mitochondria-related genes and activated mitochondria. Sirt1 is known to be involved in mitochondrial biosynthesis, and mitochondrial transcription factor A (Tfam) is a known transcription factor that functions in the mitochondria (Chandrasekaran et al. 2019). As shown in Fig. 1A and B, the supernatant derived from OS-1010-treated Caco-2 cells significantly activated Sirt1 and Tfam expression. These results showed that OS-1010 induced the secretion of secretory factors from Caco-2 cells that activate muscle cells.
OS-1010 activates muscle cells via intestinal Caco-2 cells. Culture supernatants were prepared from the Caco-2 cells treated with PBS (Control) and 100 µg/mL OS-1010. Then, 10% of the culture medium of C2C12 cells was replaced with this supernatant, and cells were further cultured for 24 h. The expression levels of Sirt1 (A) and Tfam (B) were tested by RT-PCR. Mitochondrial number (C), area (D) and activity (E) in C2C12 cells added with supernatant were evaluated using the IN Cell Analyzer 2200. The results are shown as the mean ± standard error. Multiple comparisons between groups were performed using one-way ANOVA with Tukey’s post-hoc test. Statistical significance was defined as p < 0.05 when compared to control (*p < 0.05; **p < 0.01; ***p < 0.001)
The effect of this supernatant on the mitochondria of C2C12 cells was analyzed by measuring the number, area and activity of intracellular mitochondria using mitochondria-specific fluorescent reagents (MitoTracker Red CMXRos and MitoTracker Green FM) and an IN Cell Analyzer 2200. The results showed that the supernatant did not significantly affect the number and area of mitochondria in C2C12 cells but did significantly increase mitochondrial activity (Fig. 1C–E). These results indicate that OS-1010 acts on Caco-2 cells and induces the secretion of factors that activate the mitochondria of muscle cells.
Supernatant of OS-1010-treated Caco-2 cells induced the expression of marker genes in muscle cells
Next, the effects of these supernatants on the expression of marker genes in muscle cells were analyzed. Brain derived neurotrophic factor (Bdnf) is a type of myokine secreted by muscle cells, and MyoD is a known regulator of muscle differentiation (Huang et al. 2022). As shown in Fig. 2A and B, the supernatant derived from OS-1010-treated Caco-2 cells significantly increased the expression of Bdnf and MyoD in C2C12 cells. This result indicates that the supernatant may promote C2C12 differentiation and activate cell–cell interactions through myokine secretion.
Supernatant of OS-1010-treated Caco-2 cells induced the expression of marker genes in muscle cells. Culture supernatants were prepared from the Caco-2 cells treated with PBS (Control) and 100 µg/mL OS-1010. Then, 10% of the culture medium of C2C12 cells was replaced with this supernatant, and the cells were further cultured for 24 h. The expression levels of Bdnf (A) and MyoD (B) were tested by RT-PCR. The results are shown as the mean ± standard error. Multiple comparisons between groups were performed using one-way ANOVA with Tukey’s post-hoc test. Statistical significance was defined as p < 0.05 when compared to control (*p < 0.05; **p < 0.01; ***p < 0.001)
Exosomes derived from the supernatant of OS-1010-treated Caco-2 cells activate mitochondria in C2C12 cells
The above results showed that OS-1010-treated Caco-2-derived culture supernatants activated muscle cells. Therefore, we focused on the exosomes in the supernatant and evaluated their functionality. Exosomes were prepared from the supernatant of OS-1010-treated Caco-2 cells using MagCapture Exosome Isolation Kit PS ver. 2 and their functionality against C2C12 cells was evaluated (Sugihara et al. 2019). The results showed that exosomes significantly enhanced Sirt1 expression, a gene involved in mitochondrial biosynthesis, and Tfam, a mitochondrial transcription factor, in C2C12 cells (Fig. 3A and B). Furthermore, exosomes enhanced the number, area, and activity of mitochondria in C2C12 cells (Fig. 3C–E). Unlike the supernatant, the use of exosomes clearly enhanced the effect on C2C12 cells, indicating that exosomes are potent active components secreted by OS-1010-treated Caco-2 cells.
Exosomes derived from OS-1010-treated Caco-2 cells activated C2C12 cells. Exosomes were prepared from the supernatant of Caco-2 cells treated with PBS (Control) and 100 µg/mL OS-1010. These exosomes (180 ng/mL) were added to C2C12 cells, and the cells were further cultured for 24 h. The expression levels of Sirt1 (A) and Tfam (B) were tested by RT-PCR. Mitochondrial number (C), area (D) and activity (E) in C2C12 cells added with exosomes were evaluated using the IN Cell Analyzer 2200. The results are shown as the mean ± standard error. Multiple comparisons between groups were performed using one-way ANOVA with Tukey’s post-hoc test. Statistical significance was defined as p < 0.05 when compared to control (*p < 0.05; **p < 0.01)
Exosomes derived from the supernatant of OS-1010-treated Caco-2 cells induced the expression of marker genes in muscle cells
Similarly, in the present study, exosomes derived from the supernatant of OS-1010-treated Caco-2 cells significantly enhanced the expression of Bdnf and MyoD (Fig. 4). Thus, OS-1010-treated Caco-2-derived exosomes may play an important role in the differentiation and activation of muscle cells, and these exosomes may be mediators of various cell–cell interactions that are not limited to intestinal-to-muscle interactions but originate in the gut. The reason why the results of Figs. 3 and 4 are detectable as a clear change compared to the results of Figs. 1 and 2 may be due to the difference in the amount of exosomes used: Figs. 3 and 4 use 180 ng/mL of protein equivalent exosomes, while Figs. 1 and 2 use only about 1 order of magnitude less.
Exosomes derived from OS-1010-treated Caco-2 cells induced the expression of marker genes in muscle cells. Exosomes were prepared from the supernatant of Caco-2 cells treated with PBS (Control) and 100 µg/mL OS-1010. Exosomes (180 ng/mL) were added to C2C12 cells, and the cells were further cultured for 24 h. The expression levels of Bdnf (A) and MyoD (B) were tested by RT-PCR. The results are shown as the mean ± standard error. Multiple comparisons between groups were performed using one-way ANOVA with Tukey’s post-hoc test. Statistical significance was defined as p < 0.05 when compared to control (**p < 0.01)
Molecular basis for the OS-1010-induced interaction between intestinal cells and muscle cells
The aforementioned results indicated that exosomes derived from the supernatant of OS-1010-treated Caco-2 cells activate muscle cells in terms of mitochondrial activation and augmented expression of various marker genes. We then focused on the miRNAs in exosomes derived from OS-1010-treated Caco-2 cells and identified four miRNAs whose expression was significantly altered by OS-1010 treatment (Table 1). miRNAs that showed more than 1.5-fold higher expression compared to the control treatment are shown in Table 1.
After estimating the target genes of these miRNAs, KEGG pathway analyses was performed using DAVID (Table 2). KEGG pathway analysis revealed that these miRNAs significantly affected pathways involved in the maintenance of muscle cell functionality and activation. In particular, the pathways involved in insulin signaling and cardiac muscle regulation are widely affected. Among the miRNAs analyzed, miR-8089 was found to regulate a wide range of pathways involved in the maintenance of muscle function. Activation of these pathways can result in the activation of mitochondria in muscle cells and the promotion of muscle cell differentiation, as described above. The neurotrophin signaling pathway is also activated by these miRNAs, including miR-6829-6p, miR-8089 and miR-1202. This result corresponds to the finding that OS-1010-treated Caco-2-derived supernatants and exosomes augmented Bdnf expression in C2C12 cells (Figs. 2 and 4). Furthermore, the miRNAs identified in this study were found to regulate the pathways involved in longevity, including FoxO, mTOR, and autophagy. Sirt1, which was found to be augmented in C2C12 cells by OS-1010-treated Caco-2-derived supernatants and exosomes, is also a known longevity gene, and it could be assumed that these miRNAs enhanced Sirt1 expression through the activation of the longevity signaling pathway.
Discussion
In this study, we showed that the F. fructosus OS-1010 strain, which produces high levels of NMN and NAD+, induces the secretion of exosomes from intestinal cells that can activate muscle cells. It remains to be clarified whether the NMN and NAD+ produced by OS-1010 activate intestinal cells or whether OS-1010 activates specific cell–cell interactions with intestinal cells. However, given that relatively high concentrations of NMN (500 µM) have been reported to be necessary to exert an effect on intestinal cells and to validate this effect (Ru et al. 2022), it is highly likely that the OS-1010 itself contributes to the activation of intestinal cells. These should be clarified in the future, but at least from the results of this study, it is clear that OS-1010 treatment induces the secretion of exosomes from intestinal cells that could activate muscle cells.
Our previous studies have shown that food components, such as carnosine and γ-aminobutyric acid (GABA), induce the secretion of exosomes that activate neuronal cells as a result of the activation of intestinal cells; that is, these food components can activate the gut-brain interaction via exosome secretion (Sugihara et al. 2019; Inotsuka et al. 2020, 2021). In other words, exosomes induced secretion by food components could activate interactions between organs and cells. In light of these studies, we can consider the OS-1010 strain as a new food ingredient that can activate the intestine-muscle interactions via exosomes. No reports on food ingredients that can activate intestine-muscle interactions via exosome secretion have been published, and these results indicate the possibility of a novel function for food ingredients via the intestine.
Probiotics such as lactic acid bacteria and bifidobacteria contribute to the suppression of sarcopenia, a disease muscular system, in studies using rodents. Because the skeletal muscle is physiologically distant from the intestinal tract, it is thought that intestinal-derived signals (microbial metabolites, gut peptides, lipopolysaccharides and secretory factors) resulting from intestinal stimulation contribute to the interaction between the intestinal tract and skeletal muscle and regulate muscle function (Giron et al. 2022). The involvement of intestine-derived exosomes, as revealed here, in the activation of the intestine-muscle interaction by probiotics is also expected to be clarified in the future.
References
Bolstad BM, Irizarry RA, Astrand M, Speed TP (2003) A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19:185–193. https://doi.org/10.1093/bioinformatics/19.2.185
Chandrasekaran K, Anjaneyulu M, Choi J, Kumar P, Salimian M, Ho CY, Russell JW (2019) Role of mitochondria in diabetic peripheral neuropathy: influencing the NAD(+)-dependent SIRT1-PGC-1alpha-TFAM pathway. Int Rev Neurobiol 145:177–209. https://doi.org/10.1016/bs.irn.2019.04.002
Endo A, Maeno S, Tanizawa Y, Kneifel W, Arita M, Dicks L, Salminen S (2018) Fructophilic lactic acid bacteria, a unique group of fructose-fermenting microbes. Appl Environ Microbiol. https://doi.org/10.1128/AEM.01290-18
Giron M, Thomas M, Dardevet D, Chassard C, Savary-Auzeloux I (2022) Gut microbes and muscle function: can probiotics make our muscles stronger? J Cachexia Sarcopenia Muscle 13:1460–1476. https://doi.org/10.1002/jcsm.12964
Huang DW, Sherman BT, Lempicki RA (2009a) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37:1–13. https://doi.org/10.1093/nar/gkn923
Huang DW, Sherman BT, Lempicki RA (2009b) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57. https://doi.org/10.1038/nprot.2008.211
Huang Q, Wu M, Wu X, Zhang Y, Xia Y (2022) Muscle-to-tumor crosstalk: the effect of exercise-induced myokine on cancer progression. Biochim Biophys Acta Rev Cancer 1877:188761. https://doi.org/10.1016/j.bbcan.2022.188761
Imai S (2009) The NAD World: a new systemic regulatory network for metabolism and aging–Sirt1, systemic NAD biosynthesis, and their importance. Cell Biochem Biophys 53:65–74. https://doi.org/10.1007/s12013-008-9041-4
Imai S, Guarente L (2014) NAD and sirtuins in aging and disease. Trends Cell Biol 24:464–471. https://doi.org/10.1016/j.tcb.2014.04.002
Imai S, Guarente L (2016) It takes two to tango: NAD(+) and sirtuins in aging/longevity control. NPJ Aging Mech Dis 2:16017. https://doi.org/10.1038/npjamd.2016.17
Inotsuka R, Uchimura K, Yamatsu A, Kim M, Katakura Y (2020) Gamma-aminobutyric acid (GABA) activates neuronal cells by inducing the secretion of exosomes from intestinal cells. Food Funct 11:9285–9290. https://doi.org/10.1039/d0fo01184c
Inotsuka R, Udono M, Yamatsu A, Kim M, Katakura Y (2021) Exosome-mediated activation of neuronal cells triggered by gamma-aminobutyric acid (GABA). Nutrients. https://doi.org/10.3390/nu13082544
Ishibashi A, Udono M, Sato M, Katakura Y (2023) Molecular mechanisms for the carnosine-induced activation of muscle-brain interaction. Nutrients. https://doi.org/10.3390/nu15061479
Ogawa M, Udono M, Teruya K, Uehara N, Katakura Y (2021) Exosomes derived from fisetin-treated keratinocytes mediate hair growth promotion. Nutrients. https://doi.org/10.3390/nu13062087
Poddar SK, Sifat AE, Haque S, Nahid NA, Chowdhury S, Mehedi I (2019) Nicotinamide mononucleotide: exploration of diverse therapeutic applications of a potential molecule. Biomolecules. https://doi.org/10.3390/biom9010034
Ru M, Wang W, Zhai Z, Wang R, Li Y, Liang J, Kothari D, Niu K, Wu X (2022) Nicotinamide mononucleotide supplementation protects the intestinal function in aging mice and D-galactose induced senescent cells. Food Funct 13:7507–7519. https://doi.org/10.1039/d2fo00525e
Sugihara Y, Onoue S, Tashiro K, Sato M, Hasegawa T, Katakura Y (2019) Carnosine induces intestinal cells to secrete exosomes that activate neuronal cells. PLoS ONE 14:e0217394. https://doi.org/10.1371/journal.pone.0217394
Sugiyama K, Iijima K, Yoshino M, Dohra H, Tokimoto Y, Nishikawa K, Idogaki H, Yoshida N (2021) Nicotinamide mononucleotide production by fructophilic lactic acid bacteria. Sci Rep 11:7662. https://doi.org/10.1038/s41598-021-87361-1
Yoshino J, Mills KF, Yoon MJ, Imai S (2011) Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab 14:528–536. https://doi.org/10.1016/j.cmet.2011.08.014
Author information
Authors and Affiliations
Contributions
RK performed the experiments, MU prepared figures and YK wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kashiwagi, R., Udono, M. & Katakura, Y. Fructobacillus fructosus OS-1010 strain stimulates intestinal cells to secrete exosomes that activate muscle cells. Cytotechnology 76, 209–216 (2024). https://doi.org/10.1007/s10616-023-00610-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-023-00610-1