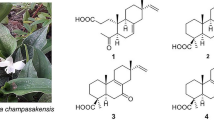
The synthesis and properties of tricyclic diterpenoids containing a C-4 functionalized oxazole ring are reported. The reaction of 4-[5-(bromomethyl)oxazol-2-yl]-18-norisopimarane with sodium azide in the presence of hydrated copper sulfate and sodium ascorbate in DMF gave the corresponding azide, from which new diterpenoid derivatives containing a 1H-substituted 1,2,3-triazol-4-yl substituent were synthesized. Reduction of the azide produced the terpenoid 5-aminomethyloxazole. Reaction of 4-[5-(bromomethyl)oxazol-2-yl]-18-norisopimarane with methyl esters of amino acids in DMF in the presence of potash synthesized compounds with oxazole C-5 amino-acid substituents. Cytotoxicity of the new isopimaric acid derivatives gainst CEM-13, MT-4, U-937, MCF-7, and MDA-MB-231 human tumor cells was studied.

Similar content being viewed by others
References
H.-Z. Zhang, Z.-L. Zhao, and C.-H. Zhou, Eur. J. Med. Chem., 144, 444 (2018).
A. Lilienkampf, J. Mao, B. Wan, Y. Wang, S. G. Franzblau, and A. P. Kozikowski, J. Med. Chem., 52, 2109 (2009).
A. Ansari, A. Alia, M. Asif, M. A. Rauf, M. Owais, and Shamsuzzaman, Steroids, 134, 22 (2018).
N. R. Jabir, C. K. Firoz, A. Bhushanb, S. Tabrez, and M. A. Kamal, Anti-Cancer Agents Med. Chem., 18, 6 (2018).
S. V. Kumar, A. Acharya, and H. Ila, J. Org. Chem., 83, 6607 (2018).
N. Rizeq and S. N. Georgiades, Molecules, 22, 2160 (2017).
M. A. Gromova, Yu. V. Kharitonov, T. V. Rybalova, and E. E. Shul′ts, Chem. Nat. Compd., 54, 293 (2018).
A. L. Spek, J. Appl. Crystallogr., 36, 7 (2003).
F. H Allen, O. Kenard, D. G. Watson, L. Bramer, A. G. Orpen, and R. Taylor, J. Chem. Soc., Perkin Trans. 2, No. 1, S1-19 (1987).
M. A. Timoshenko, A. B. Ayusheev, Yu. V. Kharitonov, M. M. Shakirov, and E. E. Shul’ts, Chem. Nat. Compd., 50, 673 (2014).
R. Grigg, V. Sridharan, M. Thornton-Pett, J. Wang, J. Xu, and J. Zhang, Tetrahedron, 58 (13), 2627 (2002).
X. Creary, A. Anderson, C. Brophy, F. Crowell, and Z. Funk, J. Org. Chem., 77, 8756 (2012).
J. K. Wilson, J. M. Sargent, A. W. Elgie, J. G. Hill, and C. G. Taylor, Br. J. Cancer, 62 (2), 189 (1990).
Yu. V. Kharitonov, E. E. Shul’ts′ and M. M. Shakirov, Chem. Nat. Compd., 49, 1067 (2014).
S. G. Davies, H. J. Sanganee, and P. Szolcsanyi, Tetrahedron, 55, 3337 (1999).
Syntheses of Organic Preparations [in Russian], No. 2, Inostrannaya Literatura, Moscow, 1949, p. 36.
G. M. Sheldrick, SADABS, Program for Area Detector Absorption Correction, Institute for Inorganic Chemistry, University of Gottingen, Germany, 1996.
G. M. Sheldrick, SHELX-97 – Programs for Crystal Structure Analysis, Release 97-2, University of Gottingen, Germany.
T. Mosmann, J. Immunol. Methods, 65 (1), 55 (1983).
H. Wan, R. Williams, P. Doherty, and D. F. Williams, J. Mater. Sci.: Mater. Med., 5 (3), 154 (1994).
Acknowledgment
The work was financially supported by grants from the RFBR (Project No. 17-43-543235) (p_mol_a) and the RSF (Project No. 18-13-00361). Biological studies were supported by Task No. 17.5484.2017/BY. Analytical and spectral studies were performed at the Chemical Research Common Use Center, NIOCh, SB, RAS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 1, January–February, 2019, pp. 47–53.
Rights and permissions
About this article
Cite this article
Gromova, M.A., Kharitonov, Y.V., Pokrovskii, M.A. et al. Synthetic Transformations of Higher Terpenoids. 37. Synthesis and Cytotoxicity of 4-(Oxazol-2-Yl)-18-Norisopimaranes. Chem Nat Compd 55, 52–59 (2019). https://doi.org/10.1007/s10600-019-02613-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-019-02613-x