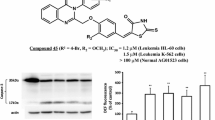
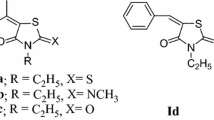
New 1,3-thiazol-2-yl-, 1,3-thiazol-4-yl-, and 1,3-thiazol-5-yl-containing derivatives of N-arylalkyl- and N-carboxyalkyl-substituted rhodanines were synthesized via Knoevenagel condensation and tested against 60 cancer cell lines of NCI panel. Among these compounds, N-[2-(4-methoxyphenyl)ethyl], N-[2-(3,4-dimethoxyphenyl)ethyl], and N-carboxydecyl rhodanines with 1,3-thiazol-4-yl or 1,3-thiazol-5-yl fragments were the most effective agents causing growth inhibition of more than 50% of the individual lines of the panel. Their activity was characterized by calculated mean values of 50% growth inhibition (GI50), total growth inhibition (TGI), and 50% cell killing (LC50). For sensitive cell lines of the total panel, mean GI50 and mean TGI were similar for compounds bearing N-[2-(4-methoxyphenyl) ethyl] and N-[2-(3,4-dimethoxyphenyl)ethyl] substituents, and significantly lower for compound with N-carboxydecyl substituent. The increase in the activity of the 1,3-thiazol-5-yl-containing derivative of N-carboxyalkyl-substituted rhodanine was accompanied by a decrease in the number of cell lines sensitive to its inhibitory effects. According to the in vitro data, some of the cancer cell lines were targeted by this compound with activity ranging from submicromolar to micromolar levels. Based on molecular docking results, the possible targets for anticancer activity of the thiazole-containing rhodanine derivatives were considered.



Similar content being viewed by others
References
Kaminskyy, D.; Kryshchyshyn, A.; Lesyk, R. Expert Opin. Drug Discovery 2017, 12, 1233.
Mermer, A. Mini-Rev. Med. Chem. 2021, 21, 738.
Mousavi, S. M.; Zarei, M.; Hashemi, S. A.; Babapoor, A.; Amani, A. M. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1132.
Szczepański, J.; Tuszewska, H.; Trotsko, N. Molecules 2022, 27, 3750.
Yin, L. J.; bin Ahmad Kamar, A. K. D.; Fung, G. T.; Liang, C. T.; Avupati, V. R. Biomed. Pharmacother. 2022, 145, 112406.
Tomasic, T.; Mašič, P. L. Expert Opin. Drug Discovery 2012, 7, 549.
Mendgen, T.; Steuer, C.; Klein, C. D. J. Med. Chem. 2012, 55, 743.
Gilberg, E.; Gütschow, M.; Bajorath, J. J. Med. Chem. 2018, 61, 1276.
Bajorath, J. Expert Opin. Drug Discovery 2021, 16, 719.
El-Mawgoud, H. K. A. Chem. Pharm. Bull. 2019, 67, 1314.
Vatolin, S.; Phillips, J. G.; Jha, B. K.; Govindgari, S.; Hu, J.; Grabowski, D.; Parker, Y.; Lindner, D. J.; Zhong, F.; Distelhorst, C. W.; Smith, M. R.; Cotta, C.; Xu, Y.; Chilakala, S.; Kuang, R. R.; Tall, S.; Reu, F. J. Cancer Res. 2016, 75, 3340.
Bernardo, P. H.; Sivaraman, T.; Wan, K.-F.; Xu, J.; Krishnamoorthy, J.; Song, C. M.; Tian, L.; Chin, J. S. F.; Lim, D. S. W.; Mok, H. Y. K.; Yu, V. C.; Tong, J. C.; Chai, C. L. L. Pure Appl. Chem. 2011, 83, 723.
Li, P.; Zhang, W.; Jiang, H.; Li, Y.; Dong, C.; Chen, H.; Zhang, K.; Du, Z. Med. Chem. Commun. 2018, 9, 1194.
Insuasty, A.; Ramírez, J.; Raimondi, M.; Echeverry, C.; Quiroga, J.; Abonia, R.; Nogueras, M.; Cobo, J.; Rodríguez, M. V.; Zacchino, S. A.; Insuasty, B. Molecules 2013, 18, 5482.
De Oliveira, J. F.; Lima, T. S.; Vendramini-Costa, D. B.; de Lacerda Pedrosa, S. C. B.; Lafayette, E. A.; da Silva, R. M. F.; de Almeida, S. M. V.; de Moura, R. O.; Ruiz, A. L. T. G.; de Carvalho, J. E.; do Carmo Alves de Lima, M. Eur. J. Med. Chem. 2017, 136, 305.
Holota, S.; Kryshchyshyn, A.; Derkach, H.; Trufin, Y.; Demchuk, I.; Gzella, A.; Grellier, P.; Lesyk, R. Bioorg. Chem. 2019, 86, 126.
Petrou, A.; Fesatidou, M.; Geronikaki, A. Molecules 2021, 26, 3166.
Sharma, P. C.; Bansal, K. K.; Sharma, A.; Sharma, D.; Deep, A. Eur. J. Med. Chem. 2020, 188, 112016.
Arshad, M. F.; Alam, A.; Alshammari, A. A.; Alhazza, M. B.; Alzimam, I. M.; Alam, M. A.; Mustafa, G.; Ansari, M. S.; Alotaibi, A. M.; Alotaibi, A. A.; Kumar, S.; Asdaq, S. M. B.; Imran, M.; Deb, P. K.; Venugopala, K. N.; Jomah, S. Molecules 2022, 27, 3994.
Ozen, C.; Unlusoy, M. C.; Aliary, N.; Ozturk, M.; Dundar, O. B. J. Pharm. Pharm. Sci. 2017, 20, 415.
Bataille, C. J. R.; Brennan, M. B.; Byrne, S.; Davies, S. G.; Durbin, M.; Fedorov, O.; Huber, K. V. M.; Jones, A. M.; Knapp, S.; Liu, G.; Nadali, A.; Quevedo, C. E.; Russell, A. J.; Walker, R. G.; Westwood, R.; Wynne, G. M. Bioorg. Med. Chem. 2017, 25, 2657.
Sing, W. T.; Lee, C. L.; Yeo, S. L.; Lim, S. P.; Sim, M. M. Bioorg. Med. Chem. Lett. 2001, 11, 91.
Holota, S.; Komykhov, S.; Sysak, S.; Gzella, A.; Cherkas, A.; Lesyk, R. Molecules 2021, 26, 1162.
Maccari, R.; Ottanà, R.; Curinga, C.; Vigorita, M. G.; Rakowitz, D.; Steindl, T. H.; Langer, T. H. Bioorg. Med. Chem. 2005, 13, 2809.
Serafini, M.; Cargnin, S.; Massarotti, A.; Pirali, T.; Genazzani, A. A. J. Med. Chem. 2020, 63, 10170.
Kobzar, O. L.,; Sinenko, V. O.; Shulha, Yu. V.; Buldenko, V. M.; Hodyna, D. M.; Pilyo, S. G.; Brovarets, V. S.; Vovk, A. I. Ukr. Bioorg. Acta. 2020, 15, 33.
Wang, H.; Hammoudeh, D. I.; Follis, A. V.; Reese, B. E.; Lazo, J. S.; Metallo, S. J.; Prochownik, E. V. Mol. Cancer Ther. 2007, 6, 2399.
Ahn, J. H.; Kim, S. J.; Park, W. S.; Cho, S. Y.; Ha, J. D.; Kim, S. S.; Kang, S. K.; Jeong, D. G.; Jung, S.-K.; Lee, S.-H.; Kim, H. M.; Park, S. K.; Lee, K. H.; Lee, C. W.; Ryu, S. E.; Choi, J.-K. Bioorg. Med. Chem. Lett. 2006, 16, 2996.
Russell, A. J.; Westwood, I. M.; Crawford, M. H. J.; Robinson, J.; Kawamura, A.; Redfield, C.; Laurieri, N.; Lowe, E. D.; Davies, S. G.; Sim, E. Bioorg. Med. Chem. 2009, 17, 905.
Sawaguchi, Y.; Yamazaki, R.; Nishiyama, Y.; Sasai, T.; Mae, M.; Abe, A.; Yaegashi, T.; Nishiyama, H.; Matsuzaki, T. Anticancer Res. 2017, 37, 4051.
Ozer, E. B.; Caglayan, C.; Bayindir, S. Tetrahedron 2022, 120, 132896.
Swain, B.; Khan, A.; Singh, P.; Marde, V. S.; Angeli, A.; Chinchilli, K. K.; Yaddanapudi, V. M.; Carradori, S.; Supuran, C. T.; Arifuddin, M. Molecules 2022, 27, 8028.
Cutshall, N. S.; O'Day, C.; Prezhdo, M. Bioorg. Med. Chem. Lett. 2005, 15, 3374.
Harada, K.; Kubo, H.; Tanaka, A.; Nishioka, K. Bioorg. Med. Chem. Lett. 2012, 22, 504.
Fu, H.; Hou, X.; Wang, L.; Dun, Y.; Yang, X.; Fang, H. Bioorg. Med. Chem. Lett. 2015, 25, 5265.
Bernardo, P. H.; Sivaraman, T.; Wang, K.-F.; Xu, J.; Krishnamoorthy, J.; Song, C. M.; Tian, L.; Chin, J. S. F.; Lim, D. S. W.; Mok, H. Y. K.; Yu, V. C.; Tong, J. C.; Chai, C. L. L. J. Med. Chem. 2010, 53, 2314.
Zhang, L.; Zhang, H.; Zhao, Y.; Li, Z.; Chen, S.; Zhai, J.; Chen, Y.; Xie, W.; Wang, Z.; Li, Q.; Zheng, X.; Hu, X. FEBS Lett. 2013, 587, 3681.
Maccari, R.; Corso, A. D.; Paoli, P.; Adornato, I.; Lori, G.; Balestri, F.; Cappiello, M.; Naß, A.; Wolber, G.; Ottanà, R. Bioorg. Med. Chem. Lett. 2018, 28, 3712.
Sun, L.; Wang, P.; Xu, L.; Gao, L.; Li, J.; Piao, H. Bioorg. Med. Chem. Lett. 2019, 29, 1187.
Xie, Y.; Liu, Y.; Gong, G.; Rinderspacher, A.; Deng, S.-X.; Smith, D. H.; Toebben, U.; Tzilianos, E.; Branden, L.; Vidović, D.; Chung, C.; Schürer, S.; Tautz, L.; Landry, D. W. Bioorg. Med. Chem. Lett. 2008, 18, 2840.
Brahmbhatt, H.; Oppermann, S.; Osterlund, E. J.; Leber, B.; Andrews, D. W. Clin. Cancer Res. 2015, 21, 2671.
Lessene, G.; Czabotar, P. E.; Sleebs, B. E.; Zobel, K.; Lowes, K. N.; Adams, J. M.; Baell, J. B.; Colman, P. M.; Deshayer, K.; Fairbrother, W. J.; Flygare, J. A.; Gibbons, P.; Kersten, W. J. A.; Kaulasegaram, S.; Moss, R. M.; Parisot, J. P.; Smith, B. J.; Street, I. P.; Yang, H.; Huang, D. C. S.; Watson, K. G. Nat. Chem. Biol. 2013, 9, 390.
Brusnakov, M.; Golovchenko, O.; Velihina, Y.; Liavynets, O.; Zhirnov, V.; Brovarets, V. ChemMedChem 2022, 17, e202200319.
Trott, O.; Olson, A. J. J. Comput. Chem. 2011, 31, 455.
Yokota, T.; Nara, Y.; Kashima, A.; Matsubara, K.; Misawa, S.; Kato, R.; Sugio, S. Proteins: Struct. Funct. Genet. 2007, 66, 272.
Murray, J. B.; Davidson, J.; Chen, I.; Davis, B.; Dokurno, P.; Graham, C. J.; Harris, R.; Jordan, A. J.; Matassova, N.; Pedder, C.; Ray, S.; Roughley, S. D.; Smith, J.; Walmsley, C.; Wang, Y.; Whitehead, N.; Williamson, D. S.; Casara, P.; Diguarher, T. L.; Hickman, J.; Stark, J.; Kotschy, A.; Geneste, O.; Hubbard, R. E. ACS Omega 2019, 4, 8892.
Wang, L.; Doherty, G. A.; Judd, A. S.; Tao, Z.-F.; Hansen, T. M.; Frey, R. R.; Song, X.; Bruncko, M.; Kunzer, A. R.; Wang, X.; Wendt, M. D.; Flygare, J. A.; Catron, N. D.; Judge, R. A.; Park, C. H.; Shekhar, S.; Phillips, D. C.; Nimmer, P.; Smith, M. L.; Tahir, S. K.; Xiao, Y.; Xue, J.; Zhang, H.; Le, P. N.; Mitten, M. J.; Boghaert, E. R.; Gao, W.; Kovar, P.; Choo, E. F.; Diaz, D.; Fairbrother, W. J.; Elmore, S. W.; Sampath, D.; Leverson, J. D.; Souers, A. J. ACS Med. Chem. Lett. 2020, 11, 1829.
Cousido-Siah, A.; Ruiz, F. X.; Crespo, I.; Porté, S.; Mitschler, A.; Pares, X.; Podjarny, A.; Farrés, J. Chem. Biol. Interact. 2015, 234, 290.
Ala, P. J.; Gonneville, L.; Hillman, M. C.; Becker-Pasha, M.; Wei, M.; Reid, B. G.; Klabe, R.; Yue, E. W.; Wayland, B.; Douty, B.; Polam, P.; Wasserman, Z.; Bower, M.; Combs, A. P.; Burn, T. C.; Hollis, G. F.; Wynn, R. J. Biol. Chem. 2006, 181, 32795.
Wei, H.; Ruthenburg, A. J.; Bechis, S. K.; Verdine, G. L. J. Biol. Chem. 2005, 280, 37041.
Berman, H. M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T. N.; Weissig, H.; Shindyalov, I. N.; Bourne, P. E. Nucleic Acids Res. 2000, 28, 235.
Sanner, M. F. J. Mol. Graph. Model. 1999, 17, 57.
Marvin 5.2.4, 2009, ChemAxon (http://www.chemaxon.com).
Hanwell, M. D.; Curtis, D. E.; Lonie, D. C.; Vandermeersch, T.; Zurek, E.; Hutchison, G. R. J. Cheminform. 2012, 4, 17.
We would like to thank National Cancer Institute, Bethesda, MD, USA, for in vitro evaluation of anticancer activity within the framework of Developmental Therapeutic Program and Enamine Ltd. for the material and technical support of the synthesis of compounds.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2023, 59(6/7), 484–493
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Los, O.V., Sinenko, V.O., Kobzar, O.L. et al. Synthesis and in vitro anticancer potential of new thiazole-containing derivatives of rhodanine. Chem Heterocycl Comp 59, 484–493 (2023). https://doi.org/10.1007/s10593-023-03220-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-023-03220-z