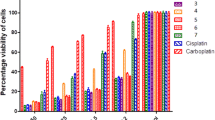
The reaction of 5-[(aminomethyl)ethynyl]thiophene-2-sulfonamides with in situ generated selenium(IV) chloride was used to synthesize 5-aminomethyl-substituted 6-chloroselenopheno[3,2-b]thiophene-2-sulfonamides. The cytotoxicity of these compounds was studied against HT-1080 (human fibrosarcoma), MH-22A (mouse hepatoma), CCL-8 (mouse sarcoma), MES-SA (human uterine sarcoma), MCF-7 (estrogen receptor-positive human breast adenocarcinoma) cell lines, as well as the normal NIH 3T3 cell line (mouse fibroblasts).
Similar content being viewed by others
References
Drake, N. Selenium: Are You Getting Enough to Reduce Your Risk of Cancer? iUniverse.com: Lincoln, 2001.
Selenium: Its Molecular Biology and Role in Human Health, 3rd ed.; Hartfield, D. L.; Berry, M. J.; Gladyshev, V. N., Eds.; Springer: New York, 2012.
Arsenyan, P.; Rubina, K.; Shestakova, I.; Domracheva, I. Eur. J. Med. Chem. 2007, 42, 635.
Shahabuddin, M. S.; Nambiar, M.; Choudhary, B.; Advirao, G. M.; Raghavan, S. C. Invest. New Drugs 2010, 28, 35.
Arsenyan, P.; Shestakova, I.; Rubina, K.; Domracheva, I.; Nesterova, A.; Vosele, K.; Pudova, O.; Lukevics, E. Eur. J. Pharmacol. 2003, 465, 229.
Arsenyan, P.; Paegle, E.; Belyakov, S.; Shestakova, I.; Jaschenko, E.; Domracheva, I.; Popelis, J. Eur. J. Med. Chem. 2011, 46, 3434.
Vasiljeva, J.; Domracheva, I.; Arsenyan, P. Tetrahedron Lett. 2016, 57, 196.
Arsenyan, P.; Vasiljeva, J.; Shestakova, I.; Domracheva, I.; Jaschenko, E.; Romanchikova, N.; Leonchiks, A.; Rudevica, Z.; Belyakov, S. C. R. Chim. 2015, 18, 399.
Mortikov, V. Y.; Litvinov, V. P.; Shestopalov, A. M.; Sharanin, Yu. A.; Apenova, E. E.; Galegov, G. A.; Abdullaev, I. I.; Asadullaev, T. B.; Abdullaev, F. I. Pharm. Chem. J. 1991, 25, 312. [Khim.-Farm. Zh. 1991, (5), 41.]
El-Bahaie, S.; Assy, M. G.; Hassanien, M. M. Pharmazie 1990, 45, 791.
Hwu, J. R.; Lai, L.-L.; Hakimelahi, G. H.; Davari, H. Helv. Chim. Acta 1994, 77, 1037.
Méplan, C.; Hesketh, J. Cancer Treat. Res. 2014, 159, 145.
Socha, K.; Kochanowicz, J.; Karpińska, E.; Soroczyńska, J.; Jakoniuk, M.; Mariak, Z.; Borawska, M. H. Nutr. J. 2014, 18, 13.
Batist, G.; Katki, A. G.; Klecker, R. W., Jr.; Myers, C. E. Cancer Res. 1986, 46, 5482.
Potapov, V. A.; Musalov, M. V.; Musalova, M. V.; Amosova, S. V. Curr. Org. Chem. 2016, 20, 136.
Potapov, V. A.; Amosova, S. V. Russ. J. Org. Chem. 2003, 39, 1373. [Zh. Org. Khim. 2003, 39, 1449.]
Potapov, V. A.; Musalov, M. V.; Abramova, E. V.; Musalova, M. V.; Rusakov, Yu. Yu.; Amosova, S. V. Chem. Heterocycl. Compd. 2014, 49, 1821. [Khim. Geterotsikl. Soedin. 2013, 1965.]
Amosova, S. V.; Martynov, A. V. Mini-Rev. Org. Chem. 2010, 7, 23.
Ninomiya, M.; Garud, D. R.; Koketsu, M. Coord. Chem. Rev. 2011, 255, 2968.
Ling, C.; Zheng, Z.; Jiang, X. C.; Zhong, W.; Li, S. Bioorg. Med. Chem. Lett. 2010, 20, 5123.
Soriano-García, M. Curr. Med. Chem. 2004, 11, 1657.
Guo, X.; Baumgarten, M.; Müllen, K. Prog. Polym. Sci. 2013, 38, 1832.
Arsenyan, P.; Oberte, K.; Pudova, O.; Lukevics, E. Chem. Heterocycl. Compd. 2002, 38, 1437. [Khim. Geterotsikl. Soedin. 2002, 1627.]
Lukevics, E.; Arsenyan, P.; Belyakov, S.; Pudova, O. Chem. Heterocycl. Compd. 2002, 38, 763. [Khim. Geterotsikl. Soedin. 2002, 867.]
Chen, H.-H.; May, J. A.; Lynch, V. M. J. Heterocycl. Chem. 1999, 36, 249.
Prugh, J. D.; Hartman, G. D.; Mallorga, P. J.; McKeever, B. M.; Michelson, S. R.; Murcko, M. A.; Schwam, H.; Smith, R. L.; Sondey, J. M.; Springer, J. P.; Sugure, M. F. J. Med. Chem. 1991, 34, 1805.
Arsenyan, P. Tetrahedron Lett. 2014, 55, 2527.
Arsenyan, P.; Vasiljeva, J.; Belyakov, S.; Liepinsh, E.; Petrova, M. Eur. J. Org. Chem. 2015, 5842.
Report of the International Workshop on in vitro Methods for Assembling Acute Systemic Toxicity; NIH Publication No.: 01-4499, 12 (2001).
This work received financial support from the Latvian Council of Science (grant No. 2012/447).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2016, 52(8), 555–558
Rights and permissions
About this article
Cite this article
Arsenyan, P., Rubina, K. & Domracheva, I. Synthesis and cytotoxicity of aminomethylselenopheno[3,2-b]thiophene sulfonamides. Chem Heterocycl Comp 52, 555–558 (2016). https://doi.org/10.1007/s10593-016-1930-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-016-1930-7