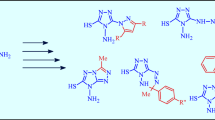
Thiomethylation of pentan-3-one, 5-methylhexan-3-one, and heptan-4-one with a mixture of formaldehyde and sodium sulfide gave the respective 3,5-dialkyltetrahydro-4H-thiopyran-4-ones, while 3,5-dihydroxymethyl- or 3,5-dialkyl-5-hydroxymethyltetrahydro-4Hthiopyran-4-ols were formed in the presence of primary amines. The oxidation of 3,5-dimethyltetrahydro-4H-thiopyran-4-one with an equivalent amount of hydrogen peroxide in tetrahydrofuran and chloroform led to the sulfoxide and sulfone, respectively, while aminomethylation with a mixture of formaldehyde and methylamine, hexylamine, monoethanolamine, or hydrochlorides of methyl, ethyl, and 2-propyl esters of aminoacetic acid at рH 7–8 produced the respective 1,5-dimethyl-3-thia-7-azabicyclo[3.3.1]nonanes. An equilibrium between the chair and boat conformations in the thiopyranone ring was found in CDCl3 solutions of 7-(2-hydroxyethyl)-1,5-dimethyl-3-thia-7-azabicyclo[3.3.1]nonan-9-one at 50°С and DMSO-d 6 solutions of 1,5,7-trimethyl-3-thia-7-azabicyclo[3.3.1]nonan-9-one monoperchlorate at 20°С.

Similar content being viewed by others
References
(а) Сhigorina, E. A.; Dotsenko, V. V. Chem. Heterocycl. Compd. 2013, 48, 1722. [Khim. Geterotsikl. Soedin. 2012, 1838.] (b) Frolov, K. A.; Dotsenko, V. V.; Krivokolysko, S. G.; Zubatyuk, R. I.; Shishkin, O. V. Chem. Heterocycl. Compd. 2013, 49, 472. [Khim. Geterotsikl. Soedin. 2013, 507.] (c) Ivanova, E. V.; Fedyanin, I. V.; Surova I. I.; Blokhin, I. V.; Atroshchenko, Yu. M.; Shahkeldyan, I. V. Chem. Heterocycl. Compd. 2013, 49, 1000. [Khim. Geterotsikl. Soedin. 2013, 1073.] (d) Vlasova, L. I.; Baibulatova, N. Z.; Dokichev, V. А.; Тоmilоv, Yu. V. Russ. Chem. Bull., Int. Ed. 2012, 61, 2129. [Izv. Akad. Nauk, Ser. Khim. 2012, 2112.]
(a) Zefirov, N. S. Usp. Khim. 1973, 45, 413. (b) Jeyaraman, R.; Avila, S. Chem. Rev. 1981, 81, 149. (c) Berlin K. D.; Tyagi, S.; Rahaman, A.; Qiu, F.; Raff, L. M.; Venkatramani, L.; Khan, M. A.; Van Der Helm, D.; Yu, V.; Praliev, K. D. Phosphorus, Sulfur Silicon Relat. Elem. 1999, 148, 97.
(a) Bailey, B. R.; Berlin, K. D.; Holt, E. M.; Scherlag, B. J.; Lazzara, R.; Brachmann, J.; Van Der Helm, D.; Powell, D. R.; Pantaleo, N. S.; Ruenitz, P. C. J. Med. Chem. 1984, 27, 758. (b) Berlin, K. D.; Scherlag, B. J.; Bailey, B. R.; Holt, E. M. US Patent 4581361. (c) Berlin, K. D.; Scherlag, B. J.; Clarke, C. R.; Otiv, S. R.; Zisman, S. A.; Sangiah, S.; Mulekar, S. V. US Patent 5084572. (d) Berlin, K. D.; Scherlag, B. J.; Clarke, C. R.; Otiv, S. R.; Zisman, S. A.; Sangiah, S.; Mulekar, S. V. US Patent 5110933. (e) Yu, V. K.; Praliev, K. D.; Fomicheva, E. E.; Mukhasheva, R. D.; Klepikova, S. G. Chem. Heterocycl. Compd. 2006, 42, 512. [Khim. Geterotsikl. Soedin. 2006, 585.]
(а) Ulendeeva, A. D.; Lyapina, N. K.; Baeva, L. A. Mercaptans in Oil and Gas Condensates [in Russian]; Izd-vo GUP INKhP RB: Ufa, 2014, p. 69. (b) Baeva, L. A. Abstract of Cand. Chem. Sci. dissertation, Ufa, 1997. (с) Ulendeeva, A. D.; Baeva, L. A.; Valiullin, O. R.; Nikitina, T. S.; Arslanova, D. D.; Spirikhin, L. V.; Lyapina, N. K. Pet. Chem. 2006, 46, 122. [Neftekhimiya 2006, 139.]
Unruh, G. R.; Birney, D. M. J. Am. Chem. Soc. 2003, 125, 8529.
Brown, М. D.; Cook, M. J.; Katritzky, A. R. J. Chem. Soc. B 1971, 2358.
Brown, М. D.; Cook, M. J.; Hutchinson, B. J.; Katritzky, A. R. Tetrahedron 1971, 27, 593.
Walker, J. F. Formaldehyde [Russian translation]; GNTI khimicheskoi literatury: Moscow, 1957, p. 261.
Klepikova, S. G.; Yu, V. K.; Fomicheva, E. E.; Mukhasheva, R. D.; Praliev, K. D.; Berlin, K. D. Chem. Heterocycl. Compd. 2008, 44, 1398. [Khim. Geterotsikl. Soedin. 2008, 1716.]
Wolfe, S.; Schlegel, H. B.; Whangbo, M.-H.; Bernardi, F. Can. J. Chem. 1974, 52, 3787.
Klimova, V. A. Basic Micromethods for the Analysis of Organic Compounds [in Russian]; Khimiya: Moscow, 1967, p. 101.
Rubinshtein, I. A.; Kleimenova, Z. A.; Sobolev, E. P. In Methods of Analysis for Organic Compounds in Oil, their Mixtures, and Derivatives [in Russian]; Gal'pern, G. D., Ed.; Izd-vo AN SSSR: Moscow, 1960, p. 74.
Tietze, L.; Eicker, T. Preparative Organic Chemistry [Russian translation]; Mir: Moscow, 1999, p. 156.
Dronov, V. I.; Nigmatullina, R. F.; Khalilov, L. M.; Nikitin, Yu. E. Zh. Org. Khim. 1985, 21, 1102.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2015, 51(11/12), 969–977
Rights and permissions
About this article
Cite this article
Baeva, L.A., Biktasheva, L.F., Fatykhov, A.A. et al. 3,5-Dialkyltetrahydro-4H-thiopyran-4-ones under the conditions of Mannich reaction. Chem Heterocycl Comp 51, 969–977 (2015). https://doi.org/10.1007/s10593-016-1806-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-016-1806-x