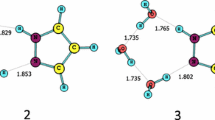
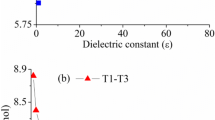
The proton transfer reactions of urazole have been investigated theoretically by using the density functional theory at the B3LYP/6-311++G(d,p) level and single-point MP2 calculations with the same basis set. The influence of the solvent on the proton transfer reactions of urazole has been examined using the self-consistent isodensity polarized continuum model in water. Also the proton transfer in the mono- and dihydrated forms of urazole have been investigated at the same level in the gas phase. It was found that the proton transfer to the carbonyl oxygen of the hydrazyl proton of urazole is the easiest in the gas phase and in water. The same proton transfer is also thermodynamically the easiest among water-assisted proton transfer reactions of urazole. In addition, the barrier heights for both H2O-assisted and (H2O)2-assisted reactions are significantly lower than those for the self-assisted tautomerization reactions of urazole.






Similar content being viewed by others
References
K. Hara, M. Kanzawa, and T. Yoshida, J. Pyrotech., 4, 15 (1996).
J. C. Oxley, J. L. Smith, Z. Zhou, and R. L. McKenny, J. Phys. Chem., 99, 10383 (1995).
C. MacLauchlin, I. H. Hall, and R.A. Izydore, Arch. Pharm., 332, 225 (1999).
I. H. Hall, K. Taylor, R. A. Izydore, D. E. Coleman, J. A. Mitchell, R. Cummings, Pharmazie, 53, 398 (1998).
I. H. Hall, O. T. Wong, R. Simlot, M. C. Miller, and R. Izydore, Anticancer Res., 12, 1355 (1992).
M.A. Hahn and R.H. Adamson, J. Nat. Cancer Inst., 48, 783 (1972).
P. F. Fox, B. M. Nash, T. J. Horan, J. O'Brien, and P. A. Morissey, J. Dairy Res., 47, 211 (1980).
Burke, T. F., Tamplar, and D. I. Curr, Curr. Ther. Res., 10, 335 (1968).
J. Perthuis and P. Poisson, FR Pat Appl. 2569712.
T. Shigematsu, H. Aizawa, T. Shibahara, M. Nakazawa, M. Tomita, and M. Tsuda, JPS Pat. Appl. 53018571.
V. M. Kolb, J. P. Dworkin, and S. L. Miller, J. Mol. Evol., 38, 549 (1994).
M. J. Bausch, B. David, and P. Dobrowolski, V. Prasad, J. Org. Chem. , 55, 5806 (1990).
J. O. Jensen, Spectrochim. Acta, Part A, 59, 637 (2003).
F. Belaj, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 48, 1088 (1992).
J. Bourdais, F. Cugniet, and J. C. Prin, P. Chabrier, Bull. Soc. Chim. Fr., 500 (1964).
E. Nachbaur, G. Faleschini, F. Belaj, and R. Janoschek, Angew. Chem., 100, 703 (1988).
M. J. Bausch, B. David, P. Dobrovolski, C. Guadalupe-Fasano, R. Gostowski, D. Selmarten, V. Prasad, A. Vaughn, and L. H. Wang, J. Org. Chem., 56, 5643 (1991).
S. Yoshino and A. Miyake, J. Therm. Anal. Calorim., 99, 145 (2010).
J. P. Ryall, T. J. Dines, B. Z. Chowdhry, S. A. Leharne, and R. Withnall, Spectrochim. Acta, Part A, 78, 918 (2011).
A. D. Becke, J. Chem. Phys., 98, 5648 (1993).
O. O. Brovarets and D. M. Hovorun, J. Biomol. Struct. Dyn., 32, 1474 (2014).
O. O. Brovarets and D. M. Hovorun, J. Biomol. Struct. Dyn., (2013). DOI: 10.1080/07391102.2013.852133.
O. O. Brovarets and D. M. Hovorun, J. Biomol. Struct. Dyn., 32, 127 (2014).
C. Møller and M. Plesset, Note on an Approximation Treatment for Many-Electron Systems, Phys. Rev., 46, 618 (1934).
C. Peng, P. Y. Ayala, H. B. Schlegel, and M. J. Frisch, J. Comput. Chem., 17, 49 (1996).
J. B. Foresman, T. A. Keith, K. B. Wiberg, J. Snoonian, and M. J. Frisch, J. Phys. Chem., 100, 16098 (1996).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, Jr., T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez, and J. A. Pople, Gaussian 03, Revision C.02, Gaussian, Inc., Pittsburgh, 2003.
E. D. Glendening, A. E. Reed, J. A. Carpenter, and F. Weinhold, NBO, Version 3.1. http://nbo6.chem.wisc.edu/
A. Furmanchuk, O. Isayev, L. Gorb, O. V. Shishkin, D. M. Hovorun, and J. Leszczynski, Phys. Chem. Chem. Phys., 13, 4311 (2011).
L.Gorb and J. Leszcynski, J. Am. Chem. Soc., 120, 5024 (1998).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 7, pp. 1069-1082, July, 2014.
Rights and permissions
About this article
Cite this article
Erdoğan, Ş., Işın, D.Ö. Theoretical Study on the Self- and Water-Assisted Proton Transfer Reaction of Urazole . Chem Heterocycl Comp 50, 986–997 (2014). https://doi.org/10.1007/s10593-014-1554-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-014-1554-8