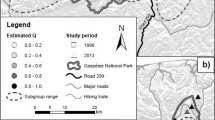
Abstract
Habitat fragmentation and the associated reduction in connectivity between habitat patches are commonly cited causes of genetic differentiation and reduced genetic variation in animal populations. We used eight microsatellite markers to investigate genetic structure and levels of genetic diversity in a relict population of wood frogs (Lithobates sylvatica) in Rocky Mountain National Park, Colorado, where recent disturbances have altered hydrologic processes and fragmented amphibian habitat. We also estimated migration rates among subpopulations, tested for a pattern of isolation-by-distance, and looked for evidence of a recent population bottleneck. The results from the clustering algorithm in Program STRUCTURE indicated the population is partitioned into two genetic clusters (subpopulations), and this result was further supported by factorial component analysis. In addition, an estimate of F ST (F ST = 0.0675, P value <0.0001) supported the genetic differentiation of the two clusters. Estimates of migration rates among the two subpopulations were low, as were estimates of genetic variability. Conservation of the population of wood frogs may be improved by increasing the spatial distribution of the population and improving gene flow between the subpopulations. Construction or restoration of wetlands in the landscape between the clusters has the potential to address each of these objectives.




Similar content being viewed by others
References
Alford RA, Richards SJ (1999) Global amphibian declines: a problem in applied ecology. Annu Rev Ecol Syst 30:133–165
Allentoft ME, Siegismund HR, Briggs L, Andersen LW (2009) Microsatellite analysis of the natterjack toad (Bufo calamita) in Denmark: populations are islands in a fragmented landscape. Conserv Genet 10:15–28
Andersen LW, Fog K, Damgaard C (2004) Habitat fragmentation causes bottlenecks and inbreeding in the European tree frog (Hyla arborea). Proc R Soc Lond B 271:1293–1302
Arens P, van der Sluis T, van’t Westende WPC, Vosman B, Vos CC, Smulders MJM (2007) Genetic population differentiation and connectivity among fragmented Moor frog (Lithobates arvalis) populations in The Netherlands. Landsc Ecol 22:1489–1500
Baker BW (2003) Beaver (Castor canadensis) in heavily-browsed environments. Lutra 46:173–181
Baldwin RF, Calhoun AJK, deMaynadier PG (2006) Conservation planning for amphibian species with complex habitat requirements: a case study using movements and habitat selection of the wood frog Lithobates sylvatica. J Herpetol 40:443–454
Beebee TJC, Rowe G (2000) Microsatellite analysis of natterjack toad Bufo calamita Laurenti populations: consequences of dispersal from a Pleistocene refugium. Biol J Linn Soc 69:367–381
Belkhir K, Borsa P, Chikli L, Raufaste N, Bonhomme F (2004) GENETIX 4.05.2, logiciel sous Windows TM pour la génétique des populations. Laboratoire Génome, Université Montpellier, Montpellier
Berven KA (1995) Population regulation in the wood frog, Lithobates sylvatica, from three diverse geographic localities. Aust J Ecol 20:292–385
Boutin-Ganache I, Raposo M, Raymond M, Deschepper CF (2001) M-13 tailed primers improve the readability and usability of microsatellite analyses performed with two different allele-sizing methods. Biotechnology 31:24–28
Chimner RA (2000) Carbon dynamics of southern rocky mountain fens. Colorado State University, Dissertation
Clayton JA, Westbrook CJ (2008) The effect of the grand ditch on the abundance of benthic invertebrates in the Colorado River, Rocky Mountain National Park. River Res Appl 24:975–987
Colorado Division of Wildlife (2005) Colorado’s comprehensive wildlife conservation strategy. Colorado Division of Wildlife, Denver
Corn PS, Livo LJ (1989) Leopard frog and wood frog reproduction in Colorado and Wyoming. Northwest Nat 70:1–9
Corn PS, Jennings ML, Muths E (1997) Survey and assessment of amphibian populations in Rocky Mountain National Park. Northwest Nat 78:34–55
Cornuet JM, Luikart G (1996) Description and power analysis of two tests for detecting recent population bottlenecks from allele frequency data. Genet 144:2001–2014
Crosby MKA, Licht LE, Fu J (2009) The effect of habitat fragmentation on finescale population structure of wood frogs (Lithobates sylvatica). Conserv Genet 10:1707–1718
Cushman SA (2006) Effects of habitat loss and fragmentation on amphibians: a review and prospectus. Biol Conserv 128:231–240
deMaynadier PG, Hunter ML Jr (1999) Forest canopy closure and juvenile emigration by pool-breeding amphibians in Maine. J Wildl Manag 63:441–450
Earl D, vonHolt BM (2011) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Res. doi:10.1007/s12686-011-9548-7
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Ficetola GF, Garner TWJ, DeBernardi F (2007) Genetic diversity, but not hatching success, is jointly affected by postglacial colonization and isolation in the threatened frog, Lithobates latastei. Mol Ecol 16:1787–1797
Ford AT, Fahrig L (2008) Movement patterns of eastern chipmunks (Tamias striatus) near roads. J Mammal 89:895–903
Frankham R (2006) Genetics and landscape connectivity. In: Crooks KR, Sanjayan M (eds) Connectivity conservation. Cambridge University Press, Cambridge, pp 72–96
Frankham R, Ballou JD, Briscoe DA (2002) Introduction to conservation genetics. Cambridge University Press, Cambridge
Funk WC, Greene AE, Corn PS, Allendorf FW (2005) High dispersal in a frog species suggests that it is vulnerable to habitat fragmentation. Biol Lett 1:13–16
Garza JC, Williamson EG (2001) Detection of reduction in population size using data from microsatellite loci. Mol Ecol 10:305–318
Gibbs JP (1998) Amphibian movements in response to forest edges, roads and streambeds in southern New England. J Wildl Manag 62:584–589
Grant EHC, Jung RE, Nichols JD, Hines JE (2005) Double-observer approach to estimating egg mass abundance of pool-breeding amphibians. Wetl Ecol Manag 13:305–320
Green DM (2003) The ecology of extinction: population fluctuation and decline in amphibians. Biol Conserv 111:331–343
Hammerson GA (1999) Amphibians and reptiles in Colorado. University Press of Colorado, Niwot
Jakobsson M, Rosenberg NA (2007) CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics 23:1801–1806
Johansson M, Primmer CR, Merila J (2007) Does habitat fragmentation reduce fitness and adaptability? A case study of the common frog (Lithobates temporaria). Mol Ecol 16:2693–2700
Julian SE, King TL (2003) Novel tetranucleotide microsatellite DNA markers for the wood frog, Lithobates sylvatica. Mol Ecol Notes 3:256–258
Kahn NW, St. John J, Quinn TW (1998) Chromosome-specific intron size differences in the avian CHD gene provide an efficient method for sex identification in birds. Auk 115:1074–1078
Lee-Yaw JA, Irwin JT, Green DM (2008) Postglacial range expansion from northern refugia by the wood frog, Lithobates sylvatica. Mol Ecol 17:867–884
Marsh DM, Fegraus EH, Harrison S (1999) Effects of breeding pond isolation on the spatial and temporal dynamics of pond use by the tungara frog, Physalaemus pustulosus. J Anim Ecol 68:804–814
McKee TB, Doesken NJ, Kleist J (1999) Historical dry and wet periods in Colorado. Climatology report 99-1, Part A: technical report, Part B. Colorado State University, Fort Collins
Miller MP (2005) Alleles in space: computer software for the joint analysis of inter-individual spatial and genetic information. J Hered 96:722–724
Newman RA, Squire T (2001) Microsatellite variation and fine-scale population structure in the wood frog (Lithobates sylvatica). Mol Ecol 10:1087–1100
Noel S, Ouellett M, Galois P, Lapointe FJ (2007) Impact of urban fragmentation on the genetic structure of the eastern red-backed salamander. Conserv Genet 8:599–606
Paetkau D, Calvert W, Stirling I, Strobeck C (1995) Microsatellite analysis of population-structure in Canadian polar bears. Mol Ecol 4:347–354
Paetkau D, Slade R, Burden M, Estoup A (2004) Genetic assignment methods for the direct, real-time estimation of migration rate: a simulation-based exploration of accuracy and power. Mol Ecol 13:55–65
Pechmann JHK, Wilbur HM (1994) Putting declining amphibian populations in perspective: natural fluctuations and human impacts. Herpetologica 50:65–84
Pechmann JHK, Scott DE, Semlitsch RD, Caldwell JP, Vitt LJ, Gibbons JW (1991) Declining amphibian populations: the problem of separating human impacts from natural fluctuations. Sci 253:892–895
Peinetti HR, Kalkhan MA, Coughenour MB (2002) Long-term changes in willow spatial distribution on the elk winter range of Rocky Mountain National Park (USA). Landscape Ecol 17:341–354
Pepin DM, Poff NL, Baron JS (2002) Ecological effects of resource development in running waters. In: Baron JS (ed) Rocky mountain futures: an ecological perspective. Island Press, Washington, DC, pp 113–132
Perry PJ (2008) It happened in Rocky Mountain National Park. Morris Book Publishing, Kearney
Petranka JW, Smith CK, Scott AF (2004) Identifying the minimal demographic unit for monitoring pond-breeding amphibians. Ecol Appl 14:1065–1078
Piry S, Alapetite A, Cornuet J-M, Paetkau D, Baudouin L, Estoup A (2004) GeneClass2: a software for genetic assignment and first-generation migrant detection. J Hered 95:536–539
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genet 155:945–959
Pruett CL, Patten MA, Wolfe DH (2009) Avoidance behavior by prairie grouse: implications for development of wind energy. Conserv Biol 23:1523–1529
Raymond M, Rousset F (1995) GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86:248–249
Regosin JV, Windmiller BS, Homan RN, Reed JM (2005) Variation in terrestrial habitat use by four pool-breeding amphibian species. J Wildl Manag 69:1481–1493
Rosenberg NA (2002) Distruct: a program for the graphical display of structure results. http://www.cmb.usc.edu/~noahr/distruct.html
Salas D, Stevens J, Schulz K (2005) Rocky Mountain National Park, Colorado: 2001–2005 vegetation classification and mapping. Technical Memorandum 8260-05-02. U.S. Bureau of Reclamation, Denver
Scherer RD (2008) Detection of wood frog egg masses and implications for monitoring amphibian populations. Copeia 2008:669–672
Scherer RD, Muths E, Noon BR (2012) The importance of local and landscape-scale processes to the occupancy of wetlands by pond-breeding amphibians. Popul Ecol. doi:10.1007/s10144-012-0324-7
Schneider S, Roessli D, Excoffier L (2000) ARLEQUIN ver 2.000: a software population genetics data analysis. Genetics and Biometry Laboratory, University of Geneva, Switzerland
Schwartz MK, Luikart G, Waples RS (2006) Genetic monitoring as a promising tool for conservation and management. Trends Ecol Evol 22:25–33
Shepard DB, Kuhns AR, Dreslik MJ, Phillips CA (2008) Roads as barriers to animal movement in fragmented landscapes. Anim Conserv 11:288–296
Smith MA, Green DM (2005) Dispersal and the metapopulation paradigm in amphibian ecology and conservation: Are all amphibian populations metapopulations? Ecography 28:110–128
Spear SF, Storfer A (2008) Landscape genetic structure of coastal tailed frogs (Ascaphus truei) in protected vs. managed forests. Mol Ecol 17:4642–4656
Squire T, Newman RA (2002) Fine-scale population structure in the wood frog (Lithobates sylvatica) in a northern woodland. Herpetologica 58:119–130
Stebbins RC (2003) A field guide to western reptiles and amphibians, 3rd edn. Houghton Mifflin Company, New York
Vasconcelos D, Calhoun AJK (2004) Movement patterns of adult and juvenile Lithobates sylvatica (LeConte) and Ambystoma maculatum (Shaw) in three restored seasonal pools in Maine. J Herpetol 38:551–561
Westbrook CJ, Cooper DJ, Baker BW (2006) Beaver dams and overbank floods influence groundwater-surface water interactions of a Rocky Mountain riparian area. Water Resour Res. doi:10.1029/2005WR004560
Weyrauch SL, Grubb TC Jr (2006) Effects of the interaction between genetic diversity and UV-B radiation on wood frog fitness. Conserv Biol 20:802–810
Wilson GA, Rannala B (2003) Bayesian inference of recent migration rates using multilocus genotypes. Genetics 163:1177–1191
Woods SW (2000) Hydrologic effects of the Grand Ditch on streams and wetlands in Rocky Mountain National Park. Thesis, Colorado State University, Colorado
Woods SW (2001) Ecohydrology of subalpine wetlands in the Kawuneeche Valley, Rocky Mountain National Park, Colorado. Dissertation, Colorado State University, Colorado
Wyoming Game and Fish Department (2005) A comprehensive wildlife conservation strategy for Wyoming. Wyoming Game and Fish Department, Cheyenne
Acknowledgments
The authors thank M. B. Albrechtsen and C. Knopf for collecting samples. Funding for this project was provided by the USGS Amphibian Research and Monitoring Initiative, Denver University’s Partners in Scholarship, and Rocky Mountain National Park. The methods in this study were approved by Colorado State University’s Animal Care and Use Committee (permit numbers 05-012A-01). Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. This is contribution number 407 of the U.S. Geological Survey Amphibian Research and Monitoring Initiative (ARMI).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scherer, R.D., Muths, E., Noon, B.R. et al. The genetic structure of a relict population of wood frogs. Conserv Genet 13, 1521–1530 (2012). https://doi.org/10.1007/s10592-012-0395-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-012-0395-1