Abstract
Imprinted X chromosome inactivation (iXCI) balances the expression of X-linked genes in preimplantation embryos and extraembryonic tissues in rodents. Long noncoding Xist RNA drives iXCI, silencing genes and recruiting Xist-dependent chromatin repressors. Some domains on the inactive X chromosome include repressive modifications specific to constitutive heterochromatin, which show no direct link to Xist RNA. We explored the relationship between Xist RNA and chromatin silencing during iXCI in vole Microtus levis. We performed locus-specific activation of Xist transcription on the only active X chromosome using the dCas9-SAM system in XO vole trophoblast stem cells (TSCs), which allow modeling iXCI events to some extent. The artificially activated endogenous vole Xist transcript is truncated and restricted ~ 6.6 kb of the exon 1. Ectopic Xist RNA accumulates on the X chromosome and recruits Xist-dependent modifications during TSC differentiation, yet is incapable by itself repressing X-linked genes. Transcriptional silencing occurs upon ectopic Xist upregulation only when repressive marks spread from the massive telomeric constitutive heterochromatin to the X chromosome region containing genes. We hypothesize that the Xist RNA-induced propagation of repressive marks from the constitutive heterochromatin could be a mechanism involved in X chromosome inactivation.







Similar content being viewed by others
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Abbreviations
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- FISH:
-
Fluorescence in situ hybridization
- H3K27me3:
-
Histone H3 trimethylated at lysine K27
- H3K4me2:
-
Histone H3 dimethylated at lysine K4
- H3K9me3:
-
Histone H3 trimethylated at lysine K9
- H4K20me3:
-
Histone H4 trimethylated at lysine K20
- PCR:
-
Polymerase chain reaction
- SAM system:
-
Synergistic Activation Mediators system
- TSC:
-
Trophoblast stem cells
- XCI:
-
X chromosome inactivation
- rXCI:
-
Random X chromosome inactivation
- iXCI:
-
Imprinted X chromosome inactivation
- Xi:
-
Inactive X chromosome
References
Borensztein M, Syx L, Ancelin K et al (2017) Xist-dependent imprinted X inactivation and the early developmental consequences of its failure. Nat Struct Mol Biol 24:226–233. https://doi.org/10.1038/nsmb.3365
Borsani DG, Tonlorenzi R, Simmler M et al (1991) Characterization of a murine gene expressed from the inactive X chromosome. Nature 351:325–329. https://doi.org/10.1038/351325A0
Brideau NJ, Coker H, Gendrel A-V et al (2015) Independent mechanisms target SMCHD1 to trimethylated histone H3 lysine 9-modified chromatin and the inactive X chromosome. Mol Cell Biol 35:4053–4068. https://doi.org/10.1128/MCB.00432-15
Brockdorff N, Ashworth A, Kay GF et al (1991) (1991) Conservation of position and exclusive expression of mouse Xist from the inactive X chromosome. Nat 3516324(351):329–331. https://doi.org/10.1038/351329a0
Calabrese JM, Sun W, Song L et al (2012) Site-specific silencing of regulatory elements as a mechanism of X inactivation. Cell 151:951–963. https://doi.org/10.1016/j.cell.2012.10.037
Chadwick BP, Willard HF (2004) Multiple spatially distinct types of facultative heterochromatin on the human inactive X chromosome. Proc Natl Acad Sci U S A 101:17450–17455. https://doi.org/10.1073/pnas.0408021101
Colognori D, Sunwoo H, Kriz AJ et al (2019) Xist deletional analysis reveals an interdependency between Xist RNA and polycomb complexes for spreading along the inactive X. Mol Cell 74:101-117.e10. https://doi.org/10.1016/j.molcel.2019.01.015
Coppola G, Pinton A, Joudrey EM et al (2008) Spatial distribution of histone isoforms on the bovine active and inactive X chromosomes. Sex Dev 2:12–23. https://doi.org/10.1159/000117715
de Napoles M, Mermoud JE, Wakao R et al (2004) Polycomb group proteins Ring1A/B link ubiquitylation of histone H2A to heritable gene silencing and X inactivation. Dev Cell 7:663–676. https://doi.org/10.1016/J.DEVCEL.2004.10.005
Dementyeva EV, Shevchenko AI, Anopriyenko OV et al (2010) Difference between random and imprinted X inactivation in common voles. Chromosoma 119:541–552. https://doi.org/10.1007/s00412-010-0277-6
Deng X, Ma W, Ramani V et al (2015) Bipartite structure of the inactive mouse X chromosome. Genome Biol 16:152. https://doi.org/10.1186/s13059-015-0728-8
Duthie SM, Nesterova TB, Formstone EJ et al (1999) Xist RNA exhibits a banded localization on the inactive X chromosome and is excluded from autosomal material in cis. Hum Mol Genet 8:195–204
Elgin SCR, Reuter G (2013) Position-effect variegation, heterochromatin formation, and gene silencing in Drosophila. Cold Spring Harb Perspect Biol 5(8):a017780. https://doi.org/10.1101/CSHPERSPECT.A017780
Elisaphenko EA, Nesterova TB, Duthie SM et al (1998) Repetitive DNA sequences in the common vole: cloning, characterization and chromosome localization of two novel complex repeats MS3 and MS4 from the genome of the East European vole Microtus rossiaemeridionalis. Chromosome Res 6:351–360. https://doi.org/10.1023/a:1009284031287
Erhardt S, Su I, Schneide R et al (2003) Consequences of the depletion of zygotic and embryonic enhancer of zeste 2 during preimplantation mouse development. Development 130:4235–4248. https://doi.org/10.1242/DEV.00625
Escamilla-Del-Arenal M, da Rocha ST, Heard E (2011) Evolutionary diversity and developmental regulation of X-chromosome inactivation. Hum Genet 130:307–327. https://doi.org/10.1007/s00439-011-1029-2
Fang J, Chen T, Chadwick BP et al (2004) Ring1b-mediated H2A ubiquitination associates with inactive X chromosomes and is involved in initiation of X inactivation. J Biol Chem 279:52812–52815. https://doi.org/10.1074/JBC.C400493200
Giorgetti L, Lajoie BR, Carter AC et al (2016) Structural organization of the inactive X chromosome in the mouse. Nature 535:575. https://doi.org/10.1038/NATURE18589
Graham FL, Smiley J, Russell WC, Nairn R (1977) Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol 36:59–72. https://doi.org/10.1099/0022-1317-36-1-59
Grigor’eva EV, Shevchenko AI, Mazurok NA et al (2009) FGF4 independent derivation of trophoblast stem cells from the common vole. PLoS One 4:e7161. https://doi.org/10.1371/journal.pone.0007161
Hines KA, Cryderman DE, Flannery KM et al (2009) Domains of heterochromatin protein 1 required for Drosophila melanogaster heterochromatin spreading. Genetics 182:967–977. https://doi.org/10.1534/GENETICS.109.105338
Hoki Y, Kimura N, Kanbayashi M et al (2009) A proximal conserved repeat in the Xist gene is essential as a genomic element for X-inactivation in mouse. Development 136:139–146. https://doi.org/10.1242/dev.026427
Ichihara S, Nagao K, Sakaguchi T, et al (2021) SmcHD1 underlies the formation of H3K9me3 blocks on the inactive X chromosome in mice. bioRxiv 2021.08.23.457321. https://doi.org/10.1101/2021.08.23.457321
Kanhere A, Viiri K, Araújo CC et al (2010) Short RNAs are transcribed from repressed polycomb target genes and interact with polycomb repressive complex-2. Mol Cell 38:675–688. https://doi.org/10.1016/j.molcel.2010.03.019
Konermann S, Brigham MD, Trevino AE et al (2015) Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature 517:583–588. https://doi.org/10.1038/nature14136
Kumaki Y, Oda M, Okano M (2008) QUMA: quantification tool for methylation analysis. 36(Web Server issue):W170-W175. https://doi.org/10.1093/nar/gkn294
Li H, Rauch T, Chen ZX et al (2006) The histone methyltransferase SETDB1 and the DNA methyltransferase DNMT3A interact directly and localize to promoters silenced in cancer cells. J Biol Chem 281:19489–19500. https://doi.org/10.1074/JBC.M513249200
Longo PA, Kavran JM, Kim M-S, Leahy DJ (2013) Transient mammalian cell transfection with polyethylenimine (PEI). Methods Enzymol 529:227–240. https://doi.org/10.1016/B978-0-12-418687-3.00018-5
Lyon MF (1961) Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature 190:372–373. https://doi.org/10.1038/190372a0
Mak W, Nesterova TB, de Napoles M et al (2004) Reactivation of the paternal X chromosome in early mouse embryos. Science 303:666–669. https://doi.org/10.1126/science.1092674
Merzouk S, Deuve JL, Dubois A et al (2014) Lineage-specific regulation of imprinted X inactivation in extraembryonic endoderm stem cells. Epigenetics Chromatin 7:11. https://doi.org/10.1186/1756-8935-7-11
Moindrot B, Brockdorff N (2016) RNA binding proteins implicated in Xist-mediated chromosome silencing. Semin Cell Dev Biol 56:58–70. https://doi.org/10.1016/J.SEMCDB.2016.01.029
Nesterova TB, Duthie SM, Mazurok NA et al (1998) Comparative mapping of X chromosomes in vole species of the genus Microtus. Chromosome Res 6:41–48
Nesterova TB, Slobodyanyuk SY, Elisaphenko EA et al (2001) Characterization of the genomic Xist locus in rodents reveals conservation of overall gene structure and tandem repeats but rapid evolution of unique sequence. Genome Res 11:833–849. https://doi.org/10.1101/gr.174901
Nozawa R-S, Nagao K, Igami K-T et al (2013) (2013) Human inactive X chromosome is compacted through a PRC2-independent SMCHD1-HBiX1 pathway. Nat Struct Mol Biol 205(20):566–573. https://doi.org/10.1038/nsmb.2532
Okamoto I, Otte AP, Allis CD et al (2004) Epigenetic dynamics of imprinted X inactivation during early mouse development. Science 303:644–649. https://doi.org/10.1126/science.1092727
Okamoto I, Patrat C, Thépot D et al (2011) Eutherian mammals use diverse strategies to initiate X-chromosome inactivation during development. Nature 472:370–374. https://doi.org/10.1038/nature09872
Orishchenko KE, Pavlova SV, Elisaphenko EA et al (2012) A regulatory potential of the Xist gene promoter in vole M. rossiaemeridionalis. PLoS One 7:e33994. https://doi.org/10.1371/journal.pone.0033994
Pinter SF (2016) A tale of two cities: how Xist and its partners localize to and silence the bicompartmental X. Semin Cell Dev Biol 56:19–34. https://doi.org/10.1016/J.SEMCDB.2016.03.023
Plath K, Fang J, Mlynarczyk-Evans SK et al (2003) Role of histone H3 lysine 27 methylation in X inactivation. Science 300:131–135. https://doi.org/10.1126/science.1084274
Prudhomme J, Dubois A, Navarro P et al (2015) A rapid passage through a two-active-X-chromosome state accompanies the switch of imprinted X-inactivation patterns in mouse trophoblast stem cells. Epigenetics Chromatin 8:52. https://doi.org/10.1186/s13072-015-0044-2
Przanowski P, Waśko U, Bhatnagar S (2018) Novel molecular players of X chromosome inactivation: new technologies and new insights. https://doi.org/10.20517/jtgg.2017.03
Rowntree RK, Lee JT (2006) Mapping of DNA replication origins to noncoding genes of the X-inactivation center. Mol Cell Biol 26:3707–3717. https://doi.org/10.1128/mcb.26.10.3707-3717.2006
Rubtsov NB, Rubtsova NV, Anopriyenko OV et al (2002) Reorganization of the X chromosome in voles of the genus Microtus. Cytogenet Genome Res 99:323–329. https://doi.org/10.1159/000071611
Schultz DC, Ayyanathan K, Negorev D et al (2002) SETDB1: a novel KAP-1-associated histone H3, lysine 9-specific methyltransferase that contributes to HP1-mediated silencing of euchromatic genes by KRAB zinc-finger proteins. Genes Dev 16:919–932. https://doi.org/10.1101/gad.973302
Sherstyuk VV, Shevchenko AI, Zakian SM (2014) Epigenetic landscape for initiation of DNA replication. Chromosoma 123:183–199. https://doi.org/10.1007/S00412-013-0448-3
Sherstyuk VV, Shevchenko AI, Zakian SM (2015) Mapping of replication origins in the X Inactivation center of vole microtus levis reveals extended replication initiation zone. PLoS ONE 10:e0128497. https://doi.org/10.1371/journal.pone.0128497
Shevchenko AI, Grigor’eva E V., Medvedev SP, et al (2018) Impact of Xist RNA on chromatin modifications and transcriptional silencing maintenance at different stages of imprinted X chromosome inactivation in vole Microtus levis. Chromosoma 127:129–139. https://doi.org/10.1007/s00412-017-0650-9
Shevchenko AI, Malakhova AA, Elisaphenko EA et al (2011) Variability of sequence surrounding the Xist gene in rodents suggests taxon-specific regulation of X chromosome inactivation. PLoS ONE 6:e22771. https://doi.org/10.1371/journal.pone.0022771
Shevchenko AI, Pavlova SV, Dementyeva EV, Zakian SM (2009) Mosaic heterochromatin of the inactive X chromosome in vole Microtus rossiaemeridionalis. Mamm Genome 20:644–653. https://doi.org/10.1007/s00335-009-9201-x
Silva J, Mak W, Zvetkova I et al (2003) Establishment of histone H3 methylation on the Inactive X chromosome requires transient recruitment of Eed-Enx1 polycomb group complexes. Dev Cell 4:481–495. https://doi.org/10.1016/S1534-5807(03)00068-6
Takagi N, Sasaki (1975) Preferential inactivation of the paternally derived X chromosome in the extraembryonic membranes of the mouse. Nature 256:640–642. https://doi.org/10.1038/256640a0
Vaskova EA, Dementyeva EV, Shevchenko AI et al (2014) Dynamics of the two heterochromatin types during imprinted X chromosome inactivation in vole Microtus levis. PLoS ONE 9:e88256. https://doi.org/10.1371/journal.pone.0088256
Vaskova EA, Medvedev SP, Sorokina AE et al (2015) Transcriptome characteristics and X-chromosome inactivation status in cultured rat pluripotent stem cells. Stem Cells Dev 24:2912–2924. https://doi.org/10.1089/scd.2015.0204
Wake N, Takagi N, Sasaki M (1976) Non-random inactivation of X chromosome in the rat yolk sac. Nature 262:580–581. https://doi.org/10.1038/262580a0
Wutz A, Jaenisch R (2000) A shift from reversible to irreversible X inactivation is triggered during ES cell differentiation. Mol Cell 5:695–705. https://doi.org/10.1016/S1097-2765(00)80248-8
Wutz A, Rasmussen TP, Jaenisch R (2002) Chromosomal silencing and localization are mediated by different domains of Xist RNA. Nat Genet 30:167–174. https://doi.org/10.1038/ng820
Zakharova IS, Shevchenko AI, Shilov AG et al (2011) Histone H3 trimethylation at lysine 9 marks the inactive metaphase X chromosome in the marsupial Monodelphis domestica. Chromosoma 120:177–183. https://doi.org/10.1007/s00412-010-0300-y
Zakian SM, Kulbakina NA, Meyer MN et al (1987) Non-random inactivation of the X-chromosome in interspecific hybrid voles. Genet Res 50:23–27. https://doi.org/10.1017/S0016672300023296
Zakian SM, Nesterova TB, Cheryaukene OV, Bochkarev MN (1991) Heterochromatin as a factor affecting X-inactivation in interspecific female vole hybrids ( Microtidae, Rodentia). Genet Res, Camb 58:105–110. https://doi.org/10.1017/S0016672300029748
Zhao J, Sun BK, Erwin JA et al (2008) Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science 322:750–756. https://doi.org/10.1126/science.1163045
Acknowledgements
The study was carried out using the resources of the Common Facilities Center of Microscopic Analysis of Biological Objects, ICG SB RAS (https://ckp.icgen.ru/ckpmabo/). We thank S.I. Bayborodin for technical assistance.
Funding
This work was supported by the State project of the Institute of Cytology and Genetics FWNR-2022–0015.
Author information
Authors and Affiliations
Contributions
AIS, SMZ, and ISZ conceived and designed the study. AIS, NAR, and ISZ conducted the experiments, analyzed data, and prepared figures. AIS and NAR wrote the manuscript. SMZ contributed to methods and models. All the authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
N/A
Consent to publish
N/A
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Irina Solovei
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 18 kb)
ESM 2
(DOCX 15 kb)
ESM 3
(DOCX 12 kb)

Supplementary Fig. 1
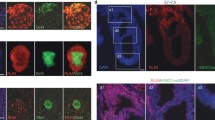
Immunofluorescent staining of metaphase spreads and nuclei with antibodies to histone modifications in R1 TSC culture (53, X) of vole M. levis (a-d). H3K4me2 is an active chromatin modification. H3K9me3, H4K20me3, a histone methyltransferase SETDB1, HP1-gamma, and KAP1protein are constitutive heterochromatin marks. Chromosomes and nuclei are counterstained with DAPI (blue). X chromosomes are indicated by arrows. MS4 is a DNA repeat specific for the X chromosome telomeric heterochromatin block in vole M. levis. Scale bar is 10 μm.(PNG 2828 kb)

Supplementary Fig. 2
Representative image of metaphase spread from clone #19 showing a diploid autosomal set and two X-chromosomes with extended Н3K9me3 distribution (green) from their constitutive heterochromatin to gene-bearing part. X chromosomes are indicated by arrows. H3K4me2 mark (red) indicates active chromatin. MS4 (white) is a DNA repeat specific for the X chromosome telomeric heterochromatin block in vole M. levis. The table shows the number and portion of metaphases with one (1X), two (2X), and three (3X) X chromosomes per a diploid autosomal set s in TSC clones #19 and #32.(PNG 995 kb)

Supplementary Fig. 3
X-linked transcripts of the Hprt1, Pgk1, Rab9, and Sb1.8 genes and Xist RNA detected by RNA FISH in vole R1 (53, X) and R2 (54, XX) TSC lines. Nuclei are counterstained with DAPI (blue). Gene transcripts are detected in red; signals from Xist RNA are in green.(PNG 2943 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shevchenko, A.I., Rifel, N.A., Zakian, S.M. et al. Constitutive heterochromatin propagation contributes to the X chromosome inactivation. Chromosome Res 30, 289–307 (2022). https://doi.org/10.1007/s10577-022-09706-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-022-09706-4