Abstract
Intracranial vascular malformations manifest on a continuum ranging from predominantly arterial to predominantly venous in pathology. Cerebral cavernous malformations (CCMs) are capillary malformations that exist at the midpoint of this continuum. The axon guidance factor Ephrin B2 and its receptor EphB4 are critical regulators of vasculogenesis in the developing central nervous system. Ephrin B2/EphB4 dysregulation has been implicated in the pathogenesis of arterial-derived arteriovenous malformations and vein-based vein of Galen malformations. Increasing evidence supports the hypothesis that aberrant Ephrin B2/EphB4 signaling may contribute to developing vascular malformations, but their role in CCMs remains largely uncharacterized. Evidence of Ephrin dysregulation in CCMs would be important to establish a common link in the pathogenic spectrum of EphrinB2/Ephb4 dysregulation. By studying patient-derived primary CCM endothelial cells (CCMECs), we established that CCMECs are functionally distinct from healthy endothelial cell controls; CCMECs demonstrated altered patterns of migration, motility, and impaired tube formation. In addition to the altered phenotype, the CCMECs also displayed an increased ratio of EphrinB2/EphB4 compared to the healthy endothelial control cells. Furthermore, whole exome sequencing identified mutations in both EphrinB2 and EphB4 in the CCMECs. These findings identify functional alterations in the EphrinB2/EphB4 ratio as a feature linking pathophysiology across the spectrum of arterial, capillary, and venous structural malformations in the central nervous system while revealing a putative therapeutic target.






Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abengozar MA, de Frutos S, Ferreiro S, Soriano J, Perez-Martinez M, Olmeda D, Marenchino M, Canamero M, Ortega S, Megias D, Rodriguez A, Martinez-Torrecuadrada JL (2012) Blocking ephrinB2 with highly specific antibodies inhibits angiogenesis, lymphangiogenesis, and tumor growth. Blood 119(19):4565–4576. https://doi.org/10.1182/blood-2011-09-380006
Akino T, Han X, Nakayama H, McNeish B, Zurakowski D, Mammoto A, Klagsbrun M, Smith E (2014) Netrin-1 promotes medulloblastoma cell invasiveness and angiogenesis, and demonstrates elevated expression in tumor tissue and urine of patients with pediatric medulloblastoma. Can Res 74(14):3716–3726. https://doi.org/10.1158/0008-5472.CAN-13-3116
Aranda E, Owen GI (2009) A semi-quantitative assay to screen for angiogenic compounds and compounds with angiogenic potential using the EA.hy926 endothelial cell line. Biol Res 42(3):377–389
Chrencik JE, Brooun A, Recht MI, Kraus ML, Koolpe M, Kolatkar AR, Bruce RH, Martiny-Baron G, Widmer H, Pasquale EB, Kuhn P (2006) Structure and thermodynamic characterization of the EphB4/Ephrin-B2 antagonist peptide complex reveals the determinants for receptor specificity. Structure 14(2):321–330. https://doi.org/10.1016/j.str.2005.11.011
Duran D, Karschnia P, Gaillard JR, Karimy JK, Youngblood MW, DiLuna ML, Matouk CC, Aagaard-Kienitz B, Smith ER, Orbach DB, Rodesch G, Berenstein A, Gunel M, Kahle KT (2018) Human genetics and molecular mechanisms of vein of Galen malformation. J Neurosurg Pediatr 21(4):367–374. https://doi.org/10.3171/2017.9.PEDS17365
Duran D, Zeng X, Jin SC, Choi J, Nelson-Williams C, Yatsula B, Gaillard J, Furey CG, Lu Q, Timberlake AT, Dong W, Sorscher MA, Loring E, Klein J, Allocco A, Hunt A, Conine S, Karimy JK, Youngblood MW, Zhang J, DiLuna ML, Matouk CC, Mane S, Tikhonova IR, Castaldi C, Lopez-Giraldez F, Knight J, Haider S, Soban M, Alper SL, Komiyama M, Ducruet AF, Zabramski JM, Dardik A, Walcott BP, Stapleton CJ, Aagaard-Kienitz B, Rodesch G, Jackson E, Smith ER, Orbach DB, Berenstein A, Bilguvar K, Vikkula M, Gunel M, Lifton RP, Kahle KT (2019) Mutations in chromatin modifier and ephrin signaling genes in vein of galen malformation. Neuron 101(3):429-443 e424. https://doi.org/10.1016/j.neuron.2018.11.041
Egea J, Klein R (2007) Bidirectional Eph-ephrin signaling during axon guidance. Trends Cell Biol 17(5):230–238. https://doi.org/10.1016/j.tcb.2007.03.004
Fehnel KP, Penn DL, Duggins-Warf M, Gruber M, Pineda S, Sesen J, Moses-Gardner A, Shah N, Driscoll J, Zurakowski D, Orbach DB, Smith ER (2020) Dysregulation of the EphrinB2-EphB4 ratio in pediatric cerebral arteriovenous malformations is associated with endothelial cell dysfunction in vitro and functions as a novel noninvasive biomarker in patients. Exp Mol Med 52(4):658–671. https://doi.org/10.1038/s12276-020-0414-0
Gault J, Sarin H, Awadallah NA, Shenkar R, Awad IA (2004) Pathobiology of human cerebrovascular malformations: basic mechanisms and clinical relevance. Neurosurgery 55(1):1–16
Goss JA, Huang AY, Smith E, Konczyk DJ, Smits PJ, Sudduth CL, Stapleton C, Patel A, Alexandrescu S, Warman ML, Greene AK (2019) Somatic mutations in intracranial arteriovenous malformations. PLoS ONE 14(12):e0226852. https://doi.org/10.1371/journal.pone.0226852
Gross BA, Lin N, Du R, Day AL (2011) The natural history of intracranial cavernous malformations. Neurosurg Focus 30(6):E24
Guclu B, Ozturk AK, Pricola KL, Bilguvar K, Shin D, O’Roak BJ, Gunel M (2005) Mutations in apoptosis-related gene, PDCD10, cause cerebral cavernous malformation 3. Neurosurgery 57(5):1008–1013
Haasdijk RA, Cheng C, Maat-Kievit AJ, Duckers HJ (2012) Cerebral cavernous malformations: from molecular pathogenesis to genetic counselling and clinical management. Eur J Hum Genet 20(2):134–140
Huang L, Nakayama H, Klagsbrun M, Mulliken JB, Bischoff J (2015) Glucose transporter 1-positive endothelial cells in infantile hemangioma exhibit features of facultative stem cells. Stem Cells 33(1):133–145. https://doi.org/10.1002/stem.1841
Kahle KT, Duran D, Smith ER (2023) Increasing precision in the management of pediatric neurosurgical cerebrovascular diseases with molecular genetics. J Neurosurg Pediatr. https://doi.org/10.3171/2022.12.PEDS22332
Kawasaki J, Aegerter S, Fevurly RD, Mammoto A, Mammoto T, Sahin M, Mably JD, Fishman SJ, Chan J (2014) RASA1 functions in EPHB4 signaling pathway to suppress endothelial mTORC1 activity. J Clin Invest 124(6):2774–2784. https://doi.org/10.1172/JCI67084
Liu J, Dong F, Jeong J, Masuda T, Lobe CG (2014) Constitutively active Notch1 signaling promotes endothelial-mesenchymal transition in a conditional transgenic mouse model. Int J Mol Med 34(3):669–676
Maddaluno L, Rudini N, Cuttano R, Bravi L, Giampietro C, Corada M, Ferrarini L, Orsenigo F, Papa E, Boulday G, Tournier-Lasserve E, Chapon F, Richichi C, Retta SF, Lampugnani MG, Dejana E (2013) EndMT contributes to the onset and progression of cerebral cavernous malformations. Nature 498(7455):492–496. https://doi.org/10.1038/nature12207
Peterson K, Coffman S, Zehri A, Anzalone A, Xiang Z, Wolfe S (2021) Somatic mosaicism in the pathogenesis of de novo cerebral arteriovenous malformations: a paradigm shift implicating the RAS-MAPK signaling cascade. Cerebrovasc Dis 50(2):231–238. https://doi.org/10.1159/000512800
Pricola Fehnel K, Duggins-Warf M, Zurakowski D, McKee-Proctor M, Majumder R, Raber M, Han X, Smith ER (2016) Using urinary bFGF and TIMP3 levels to predict the presence of juvenile pilocytic astrocytoma and establish a distinct biomarker signature. J Neurosurg Pediatr. https://doi.org/10.3171/2015.12.PEDS15448
Ren AA, Snellings DA, Su YS, Hong CC, Castro M, Tang AT, Detter MR, Hobson N, Girard R, Romanos S, Lightle R, Moore T, Shenkar R, Benavides C, Beaman MM, Muller-Fielitz H, Chen M, Mericko P, Yang J, Sung DC, Lawton MT, Ruppert JM, Schwaninger M, Korbelin J, Potente M, Awad IA, Marchuk DA, Kahn ML (2021) PIK3CA and CCM mutations fuel cavernomas through a cancer-like mechanism. Nature 594(7862):271–276. https://doi.org/10.1038/s41586-021-03562-8
Ren J, Huang Y, Ren Y, Tu T, Qiu B, Ai D, Bi Z, Bai X, Li F, Li JL, Chen XJ, Feng Z, Guo Z, Lei J, Tian A, Cui Z, Lindner V, Adams RH, Wang Y, Zhao F, Korbelin J, Sun W, Wang Y, Zhang H, Hong T, Ge WP (2023) Somatic variants of MAP3K3 are sufficient to cause cerebral and spinal cord cavernous malformations. Brain 146(9):3634–3647. https://doi.org/10.1093/brain/awad104
Seker A, Pricola KL, Guclu B, Ozturk AK, Louvi A, Gunel M (2006) CCM2 expression parallels that of CCM1. Stroke 37(2):518–523
Snellings DA, Girard R, Lightle R, Srinath A, Romanos S, Li Y, Chen C, Ren AA, Kahn ML, Awad IA, Marchuk DA (2022) Developmental venous anomalies are a genetic primer for cerebral cavernous malformations. Nat Cardiovasc Res 1:246–252. https://doi.org/10.1038/s44161-022-00035-7
Takada S, Hojo M, Tanigaki K, Miyamoto S (2017) Contribution of endothelial-to-mesenchymal transition to the pathogenesis of human cerebral and orbital cavernous malformations. Neurosurgery 81(1):176–183. https://doi.org/10.1093/neuros/nyx078
Tanriover G, Boylan AJ, Diluna ML, Pricola KL, Louvi A, Gunel M (2008) PDCD10, the gene mutated in cerebral cavernous malformation 3, is expressed in the neurovascular unit. Neurosurgery 62(4):930–938
Van Damme A, Seront E, Dekeuleneer V, Boon LM, Vikkula M (2020) New and emerging targeted therapies for vascular malformations. Am J Clin Dermatol 21(5):657–668. https://doi.org/10.1007/s40257-020-00528-w
Weng J, Yang Y, Song D, Huo R, Li H, Chen Y, Nam Y, Zhou Q, Jiao Y, Fu W, Yan Z, Wang J, Xu H, Di L, Li J, Wang S, Zhao J, Wang J, Cao Y (2021) Somatic MAP3K3 mutation defines a subclass of cerebral cavernous malformation. Am J Hum Genet 108(5):942–950. https://doi.org/10.1016/j.ajhg.2021.04.005
You C, Zhao K, Dammann P, Keyvani K, Kreitschmann-Andermahr I, Sure U, Zhu Y (2017) EphB4 forward signalling mediates angiogenesis caused by CCM3/PDCD10-ablation. J Cell Mol Med 21(9):1848–1858. https://doi.org/10.1111/jcmm.13105
Acknowledgements
Be Brave for Life, Kids@Heart Fund, Chae Fund, Irving Fund, Lucas Warner Research Fund. We thank Dr. Keith Ligon, Cecilia Sousa, and Jayne Vogelzang from the Pediatric Neuro-Oncology/Neuro-Pathology at Dana Farber Cancer Institute and the Pathology Department at Boston Children's Hospital for providing the CCM patient tissue samples. We thank Caitlin Edwards and the Brigham and Women’s Hospital Imaging Core for their assistance with the histopathology staining. We thank Psomagen for performing and analyzing the WES.
Funding
This work was funded by Be Brave for Life, Kids@Heart Fund, Chae Fund, Irving Fund, Lucas Warner Research Fund.
Author information
Authors and Affiliations
Contributions
KF and ERS: designed the study and provided funding. JS, AG, JD, and TM: conducted the experiments and acquired the data. JS, AG, and AL: analyzed the data. SA: provided the patient samples. KF, ERS, and JS: wrote and reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical Approval
This study was approved by the Boston Children’s Hospital IRB.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10571_2023_1447_MOESM1_ESM.tif
Supplementary file1 (TIF 4729 KB) Supplementary Figure 1 These images represent five different CCM patient cell lines at confluency. Images were taken on the ECHO Rebel-18 Microscope at 4X objective. Scale bar added for size determination.
10571_2023_1447_MOESM2_ESM.tif
Supplementary file2 (TIF 1696 KB) Supplementary Figure 2 Immunoblot representing the full western blots that are depicted in Fig. 4. A–C are representations of the same immunoblot with different protein expressions. A Shows the EphrinB4 band between 100 and150 kD. B Shows the Ephrin B2 band at 50 kD and the αSMA band at 42 kD. C Shows the GAPDH band at 37kD.
10571_2023_1447_MOESM3_ESM.tiff
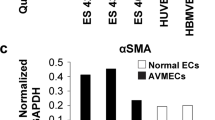
Supplementary file3 (TIFF 1045 KB) Supplementary Figure 3 Immunoblots representing the expression of CD31 and alpha-SMA in normal endothelial cells (HUVECs and HBMVEC) and in CCM patient cells. GAPDH is used as a loading control.
10571_2023_1447_MOESM4_ESM.xlsx
Supplementary file4 (XLSX 17 KB) Supplemental File 1 Analyses using nonparametric testing, the test statistic (Z), and the p-value from the Wilcoxon rank sum test are reported. Z values and p values are shown in the results tables.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sesen, J., Ghalali, A., Driscoll, J. et al. Discovery and Characterization of Ephrin B2 and EphB4 Dysregulation and Novel Mutations in Cerebral Cavernous Malformations: In Vitro and Patient-Derived Evidence of Ephrin-Mediated Endothelial Cell Pathophysiology. Cell Mol Neurobiol 44, 12 (2024). https://doi.org/10.1007/s10571-023-01447-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10571-023-01447-0